Abstract
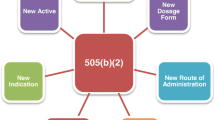
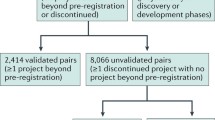
Drug repurposing refers to the development of existing drugs for new indications. These drugs may have (i) failed to show efficacy in late stage clinical trials, without safety issues; (ii) stalled in development for commercial reasons; (iii) passed the point of patent expiry; or (iv) are being explored in new geographical markets. Over the past 5 years, pressure on the industry caused by the ‘innovation gap’ of rising development costs and stagnant product output have become major reasons for the growing interest in drug repurposing. Companies have emerged offering a variety of broad platforms for identifying new indications, and some have been successful in building their own pipelines of candidates for which the risks and timelines associated with further clinical development are reduced. The business models and platforms offered by these companies will be validated if they are able to generate positive proof-of-concept clinical data for their repurposed compounds. In the current economic climate, two factors may continue the flow of compounds into repurposing projects. First, the need to expand product pipelines with new projects has become more acute, especially with projects for which an element of the risk has been removed. Second, the pool of potential compounds abandoned for strategic reasons is growing. These factors may continue to provide opportunities for academia as well as a small number of successful indications discovery companies. Moving forward, the pharma industry is looking to explore the full potential of their compounds at a much earlier stage, perhaps even in late preclinical development, offering additional opportunities for these companies.





Similar content being viewed by others
References
Pharmaceutical Research and Manufacturers of America (PhRMA) [online]. Available from URL: http://www.phrma.org/ [Accessed 2010 Jun 29]
US Food and Drug Administration [online]. Available from URL: http://www.fda.gov/ [Accessed 2010 Jun 29]
Shah S, Federoff HJ. Drug discovery dilemma and Cura quartet collaboration. Drug Discovery Today 2009 Nov 14; 21–22: 1006–10
Munos B. Lessons from 60 years of pharmaceutical innovation. Nature Reviews Drug Discovery 2009 Dec; 8: 959–68
Stuart-Kregor P. Drug repurposing. Surrey: MSI Consultancy, 2007
Cutting Edge Information. Pharmaceutical product relaunch: preserving market share through line extension and new market entry strategies. 2007 [online]. Available from URL: http://www.cuttingedgeinfo.com/productrelaunch/?overview [Accessed 2010 Jun 3]
Barratt MJ, Frail DE. Integration of indications discovery efforts at Pfizer. International Drug Discovery 2010; 5: 72–5
United States Securities and Exchange Commission: Washington D.C. 20549: form 10-Q [online]. Available from URL: http://www.sec.gov/Archives/edgar/data/1043914/000115752307008179/a5467951.htm [Accessed 2010 Jun 25]
Zerhouni E. Medicine. The NIH Roadmap. Science 2003; 302: 63–72
Schubert C. Matchmaking service links up researchers to wallflower drugs [editorial]. Nat Med 2009 Jan; 16 (1): 7
Bradley D. Why big pharma needs to learn the three Rs [editorial analysis]. Nat Rev Drug Discov 2005 June 1; 4: 446
Acknowledgements
The authors thank the following representatives of industry and academia who gave their time through interviews during the preparation of this article: Andrew Reaume, Chief Executive Officer, Melior Discovery; Jason Levin, Chief Business Officer, Brain Cells Inc.; and Kate Marusina, Manager, Research Facilitation and Industry Alliance, Clinical and Translational Science Center, University of California Davis School of Medicine.
No sources of funding were used to assist in the preparation of this article. The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sleigh, S.H., Barton, C.L. Repurposing Strategies for Therapeutics. Pharm Med 24, 151–159 (2010). https://doi.org/10.1007/BF03256811
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256811