Abstract
We tested the stability and reaction of several amino acids using hydrothermal system simulators: an autoclave and two kinds of flow reactors at 200–250 °C. This study generally showed that there is a variation in the individual amino acids survivability in the simulators. This is mainly attributed to the following factors; heat time, cold quenching exposure, metal ions and also silica. We observed that, in a rapid heating flow reactor, high aggregation and/or condensation of amino acids could occur even during a heat exposure of 2 min. We also monitored their stability in a reflow-type of simulator for 120 min at 20 min intervals. The non-hydrolyzed and hydrolyzed samples for this system showed a similar degradation only in the absence of metal ions.
Similar content being viewed by others
Introduction
Hydrothermal systems could well be a spot where the origin of life had occurred on Earth (Corliss et al. 1981) due to its high energy, reducing environment and the availability of various starting chemicals (i.e. CH4, C2H6, H2S, CO, and H2). The emergence of many biomolecules subsequently propagated to life as we know it now (Holm 1982; Wächtershäuser 1990; Shock 1992). Many experiments have been done since, to simulate this kind of prebiotic environment to produce biomolecules and/or test their survivability (Miller and Bada 1988; Kobayashi et al. 1995; Imai et al. 1999; Islam et al. 2001).
Previously, autoclaves were used as a common apparatus to perform experiments involving hydrothermal systems (e.g. Kobayashi et al. 1995; Kohara et al. 1997; Miller and Bada 1988) which was more of a closed system. A flow-type simulator was also suggested by Corliss (1986) to better represent the hydrothermal environment in an open manner together with a rapid quenching cold bath to sieve meta-stable products (Matsuno and Svenson 1999). Since then many such flow reactors were made (Matsuno 1997; Imai et al. 1999; Islam et al. 2001).
As previously mentioned, an autoclave represents a stagnant system where chemicals are heated slowly either in a glass tube or metal container at high temperature (100–400 °C). Kohara et al. (1997) demonstrated using such a system that the survivability of amino acids are higher when reducing conditions are introduced. Although autoclaves are known for their robustness and can perform experiments for a very long duration, the apparatus severely lacks a proper cooling device, which could make a difference in the chemical reactions.
A flow reactor on the other hand is a more dynamic system where chemicals are flowed to a heater and cooled down rapidly by a cooling bath. Islam et al. (2001, 2003) demonstrated this using the Super Critical Water Flow Reactor (SCWFR) utilizing an infrared (IR) gold image furnace, which enables the fluid in this system to heat up to 400 °C (or any desired temperature) within seconds without pre-heating. They showed that oligomerization of glycine could occur within 2 min of heating. Interestingly, this also could be happening in reality, where micro dikes near the vents could be venting out fluids as rapid as in 2 min. Baker et al. (1989) reported that fluid velocity at southern Juan de Fuca Ridge, was about 1–5 m/s in a 1–3 km length path. Based on this, a 3–60 min could be estimated with this model, making the system somewhat replicable in the laboratory.
A hydrothermal circulation flow reactor (abbreviated for convenience as HCFR) which is a reflow-type of system, was designed by Matsuno (1997). Oligomerization of amino acids (Imai et al. 1999; Ogata et al. 2000), nucleotides (Ogasawara et al. 2000) and even the encapsulation of oligomers within a lipid vesicle (Furuuchi et al. 2005) was achieved by this apparatus. We could postulate this scenario happening periodically near a down flow zones around the hydrothermal system.
Many spots, not necessarily the vent itself, such as open dikes near down flows and even plutons associated with the system could be simulated, where similar prebiotic chemical reactions can take place despite the constraints of their resident time. However it’s worth noting that designing a realistic simulation apparatus is rather difficult, since real hydrothermal systems are complex, very dynamic and vary from another (Kelley et al. 2002; Shock 1992).
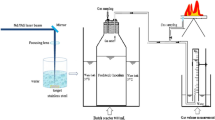
Here, we utilized an autoclave, SCWFR and the HCFR (Fig. 1) and compared the survivability of Glycine (Gly), L-Alanine (L-Ala), L-Aspartic acid (L-Asp), L-Valine (L-Val), L-Serine (L-Ser) and L-Proline (L-Pro) before and after acid hydrolysis. We conducted our experiments with parameters ranging between 200 and 250 °C at 20–25 MPa considering the typical hydrothermal environments (Kelley et al. 2002; Hazen et al. 2002; Martin et al. 2008). Although such recovery experiments have been done with the SCWFR (Islam et al. 2003) and autoclave (Kohara et al. 1997), the recovery utilizing HCFR has yet to be attained. The HCFR gives us the capacity to recover the samples at some desired time intervals (e.g. 20 min) over the entire experiment. This gives an advantage over the autoclave to monitor the chemical reactions that happens during the time range. We also tested the autoclave and HCFR with metal ions (CuCl2, MnCl2.4H2O and FeCl2.4H2O) to see their influencing effects on the overall survivability of the amino acids. Reducing conditions were compared with the SCWFR as well.
Materials and Methods
As mentioned earlier, we used three different type of apparatus to simulate hydrothermal system (Fig. 1): Autoclave, SCWFR and the HCFR. All experiments were done using milli-Q water and analytical grade chemicals. Amino acids (Gly, L-Ala, L-Ser, L-Asp, L-Val and L-Pro), CuCl2, MnCl2.4H2O and FeCl2.4H2O were purchased from Wako Pure chemicals. The gas mixture of H2(1 %) + N2(99 %) was obtained from Takachiho Kagasu. All glassware were baked at 500 °C before use.
For the autoclave experiments (See Kohara et al. 1997; Kobayashi et al 1995, 1997 for details), we used a mixture of 10 ml of 20 mM each for Gly, L-Ala, L-Ser, L-Asp, L-Val and L-Pro in a pyrex glass tube (Fig. 1a). These tubes were then placed into a 100 ml autoclave (Fig. 1a). We performed the experiments of amino acids with and without metal ions of 40 μl of 10 mM CuCl2, 0.1 mM MnCl2.4H2O and 0.1 mM FeCl2.4H2O (Yanagawa et al. 1980). We decided to perform both the autoclave experiments with 20 MPa of reducing (N2 + H2) gas as a default. Kohara et al. (1997), Kobayashi et al. (1995, 1997) have both emphasized having a high hydrogen fugacity as in the real hydrothermal systems. The autoclave uses an electrothermal heater, in which the heating starts from room temperature to the desired temperature of 200–250 °C, which takes about 30 min. We then allow the heat chamber to cool down with a fan after 120 min has elapsed.
For the SCWFR (See Islam et al. 2001, 2003; Kurihara et al. 2012 for apparatus details), similar conditions such as pressure (25 MPa) and temperature (200–230 °C) were utilized. We injected 1 ml of 20 mM of each amino acid together into the system, maintaining the flow rate of 0.5 ml/min (Fig. 1b). The injected sample pass through the IR image gold furnace for a 2 min of heat exposure. The experiments were performed with and without reducing (N2 + H2) gas conditions. The reducing gas (N2 + H2) mixture was bubbled inside the H2O reservoir to compare their effects. Metal ions were not used here due to the apparatus limitation.
The HCFR (see Matsuno et al. 1997; Imai et al. 1998; Ogata et al. 2000) represents a reflow system (Fig. 1c). We used 250 ml of 20 mM of amino acid which was flowed/reflowed at a rate of 10 ml/min giving the sample a heat time of approximately 2 min as well. All experiments were done at about 23 MPa; samples were collected at 20 min intervals for 2 h. The heat cycle time for the total volume of fluid, 250 ml, is 85 min. The electrothermal heater takes about 10 min to reach about 230 °C from room temperature, in which each run lasted for 120 min. Experiments were done with 1 ml of 10 mM CuCl2, 0.1 mM MnCl2.4H2O and 0.1 of mM FeCl2.4H2O and without metals (similar mol ratios as autoclave samples).
All products of amino acids were hydrolyzed with 6 M HCl for 24 h at 110 °C, and pass through a desalting procedure using a solid phase extraction spin column Monospin-SCX provided by GL sciences. An ion-exchange HPLC was used to analyze the sample, which is made up of two HPLC pumps (Shimadzu LC-10), a cation exchange column (Shimpak ISC-07/S1504, 4 mm i.d. × 150 mm), with post-column derivatization, and a Shimadzu RF-535 fluorometric detector. N-acetyl-L-cysteine (N-AceCys) and o-phthalaldehyde (OPA) were used as derivatizing agent in the borate buffer. NaClO (5 %) was included to enhance the detection of L-proline (Fujiwara et al. 1987). Procedure blanks was done in every steps of the experiments (simulation, hydrolysis, desalting and analysis).
Results and Discussion
Figure 2 shows the recovery percentage of amino acids before and after hydrolysis. L-Asp is showing the lowest recovery in all these apparatus. This was expected, as several studies has pointed out that L-Asp has a very low heat tolerance followed by L-Ser in simulated hydrothermal systems (Miller and Bada 1988; Kobayashi et al. 1995; Kohara et al. 1997). When compared between the three systems; the HCFR is better at conserving L-Asp possibly due to its periodic heat exposure over the span of 120 min. On the contrary L-Asp is conserved the least in SCWFR despite the amino acid being exposed for only 2 min. We could observe the reverse happening between the two systems, when L-Ser shows the highest recovery in both default and N2 + H2 experiments in the SCWFR. Similar observation are noted for the rest of the amino acids suggesting that, each individual system presents a favorable and a non uniform recovery for each amino acid. We could attribute this phenomena to the heating and cooling exposure of each system since this is the main difference in the experimental parameters. However, exactly how the systems favors an amino acid over another remains to be understood.
The recovery ratios of all hydrolyzed amino acids are often higher than the non hydrolyzed ones for all three apparatus. These results are consistent with the work of Kohara et al. (1997) and Islam et al. (2003). They suggested that amino acids do not only decompose but could condense or aggregate, resulting in a higher recovery once hydrolyzed.
With the HCFR, we sampled the amino acids at a 20 min interval for 120 min. On the 120th minute, the sample will have gone through about 2.4 cycles, making it entirely exposed to the desired heating. As shown in Fig. 3, both L-Asp and L-Ser goes through a steep decomposition rate from 20 min onwards, where we believe, they decompose to become L-Ala and/or Gly. This could also explain why Gly and L-Ala show little or no decomposition during the 20–100 min period. We assumed here that aspartic acid’s side chain could undergo decarboxylation to become alanine. However, serine decomposition reaction is more complex where it undergoes decarboxylation, dehydration followed by transamination or reduction to become alanine; and the reversible dealdolization to become glycine and formaldehyde (Bada and Miller 1970; Friedmann et al. 1971). Similar findings as such have also been reported where L-Asp decomposes followed by L-Ser and the subsequent appearance of Gly and L-Ala irrespective of their inclusion in the starting materials (Aubrey et al. 2008; Andersson and Holm 2000; Islam et al. 2003; Miller and Bada. 1988).
The inclusion of metal ions in HCFR during the 120 min show a significant difference in concentration between hydrolyzed and non hydrolyzed samples for Gly, L-Ala and L-Val and to a lesser extent, L-Asp and L-Ser (Fig. 3). On the other hand, L-Pro shows an overall high and similar recovery for both the portions. We suspect that several processes are happening when metal ions are involved. The inclusion of Cu2+, Mn2+ and Fe2+ usually increases the formation of oligomers (Imai et al. 1999 and Rode 1999). However, these transitional metals are also known to have a catalytic effect on the hydrolysis of peptide bonds as well (Leach and Angelici 1968 and Long and Truscott 1963). This could be happening when the recovery rates of the hydrolyzed and non hydrolyzed portions are similar which is observed at the 120 min mark for almost all the amino acids. Both condensation and hydrolysis could be happening in the system but to what extent remains unknown. We are also not excluding the possible formation of organometal complexes (Datta and Rabin 1956; Raju and Mathur 1968), which is rarely studied in this area of research.
We would also like to put some emphasis on the role of silica (pyrex tube) in the autoclave experiments. We noticed that Gly, L-Pro and L-Ser are showing higher recovery compared to the samples with metals. This is consistent with work done by Ito et al. (2009), where they heated up (230 °C) siliceous ooze with amino acid, to show high recovery for Gly, L-Pro and L-Ser. We could postulate that Gly’s better silica adsorption (Meng et al. 2004) and L-Ser’s hydroxyl group binding with silicate could be causing this. However there is a lack of understanding for L-Pro’s behavior with silica.
As previously mentioned, difference between the non hydrolyzed and hydrolyzed portion could mean that the amino acids are aggregating or condensing. We noticed that in the SCWFR, amino acids with and without mild reducing conditions (N2 + H2) that were flowed for 2 min shows (Fig. 2) an abrupt profile between the portions, where reduced samples show a higher recovery. The importance of having a high hydrogen fugacity is explained elsewhere (Kobayashi et al. 1997; Kohara et al. 1997 and Islam et al. 2003). Amino acids flowed through the SCWFR, are aggregating quickly within the 2 min heat time rather than decomposing, which is significantly higher compared to their 120 min exposure in the reflow system (HCFR) and the autoclave respectively. Unlike the HCFR and autoclave, the SCWFR utilized a rapid heated furnace where injected samples are exposed to a rather short and rapid heat. We believe this, together with rapid cold-quenching effect (Ogata et al. 2000; Matsuno and Swenson 1999), acted as an effective sink to preserve the amino acids aggregates, thus preventing them from further decomposition. It was previously thought that the rapid cold-quenching effect in the flow simulators (HCFR and SCWFR) could act as a sieve to preserve unstable products that could easily degrade. However, our results suggest that it is possible that a rapid heat exposure (2 min) could become a significant contributor to produce and/or conserve more biomolecules (in this case amino acid aggregates).
Conclusion
We have shown how different hydrothermal simulators give varied results for the selected amino acids. The SCWFR shows the highest change in recovery between hydrolyzed and non hydrolyzed samples, suggesting the role of short (2 min) rapid heat exposure as a possible cause for more aggregation and/or condensation. We believe that further examination of the aggregates will be beneficial as it can reveal the possible occurrence of prebiotic peptides. We also used a reflow-type flow reactor (HCFR) to know how the amino acids react in intervals over 120 min. Here, we can only speculate about some of the reactions (condensation or hydrolysis) of amino acids and metal ions. We believe that more emphasis should be given to metals or minerals and how they interact with biomolecules in hydrothermal systems; which will be more realistic and reliable for better simulation related to the origin of life on Earth and other terrestrial planets.
References
Aubrey AD, Cleaves HJ, Bada JL (2008) The role of submarine hydrothermal systems in the synthesis of amino acids. Orig Life Evol Biosph 39(2):91–108. doi:10.1007/s11084-008-9153-2
Andersson E, Holm NG (2000) The stability of some selected amino acids under attempted redox constrained hydrothermal conditions. Orig Life Evol Biosph 30(1):9–23. doi:10.1023/A:1006668322298
Bada JL, Miller SL (1970) Kinetics and mechanism of the reversible nonenzymic deamination of aspartic acid. J Am Chem Soc 92(9):2774–2782
Baker ET, Lavelle JW, Massoth GJ, Walker SL (1989) Episodic venting of hydrothermal fluids from the Juan de Fuca Ridge. J Geophys Res 94:9237–9250
Corliss JB (1986) On the creation of living cells in submarine hot spring flow reactors: attractors and bifurcations in the natural hierarchy of dissipative systems. Orig Life Evol Biosph 16(3–4):381–382. doi:10.1007/BF02422084
Corliss JB, Baross JA, Hoffman SE (1981) An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanol Acta 4(Suppl):59–69
Datta SP, Rabin BR (1956) The chelation of metal ions by dipeptides and related substances. Part 2.—Cupric complexes. Trans Faraday Soc 52:1123–1130. doi:10.1039/TF9565201123
Fujiwara M, Ishida Y, Nimura N, Toyama A, Kinoshita T (1987) Postcolumn fluorometric detection system for liquid chromatographic analysis of amino and imino acids using o-phthalaldehyde/N-acetyl-L-cysteine reagent. Anal Biochem 166(1):72–78
Furuuchi R, Imai E, Honda H, Hatori K, Matsuno K (2005) Evolving lipid vesicles in prebiotic hydrothermal environments. Orig Life Evol Biosph 35(4):333–343. doi:10.1007/s11084-005-2039-7
Friedmann N, Haverland WJ, Miller SL (1971) Prebiotic synthesis of the aromatic and other amino acids. In: Buvet R, Ponnamperuma C (eds) Chemical evolution and the origin of life. North-Holland Publishing Co, Amsterdam, pp 123–135
Hazen RM, Boctor N, Brandes JA, Cody GD, Hemley RJ, Sharma A, Yoder HS Jr (2002) High pressure and the origin of life. J Phys Condens Matter 14(44):11489
Holm NG (1982) Chapter 1 why are hydrothermal systems proposed as plausible environments for the origin of life? Orig Life Evol Biosph 22(1–4):5–14. doi:10.1007/BF01808015
Imai E, Honda H, Hatori K, Brack A, Matsuno K (1999) Elongation of oligopeptides in a simulated submarine hydrothermal system. Science 283(5403):831–833. doi:10.1126/science.283.5403.831
Imai E, Honda H, Hatori K, Matsuno K (1998) Autocatalytic synthesis of oligoglycine in a simulated submarine hydrothermal system. Orig Life Evol Biosph 29(3):249–259
Islam MN, Kaneko T, Kobayashi K (2001) Determination of amino acids formed in a supercritical water flow reactor simulating submarine hydrothermal systems. Anal Sci 17:1631–1634
Islam MN, Kaneko T, Kobayashi K (2003) Reaction of amino acids in a supercritical water-flow reactor simulating submarine hydrothermal systems. Bull Chem Soc Jpn 76(6):1171–1178. doi:10.1246/bcsj.76.1171
Ito M, Yamaoka K, Masuda H, Kawahata H, Gupta LP (2009) Thermal stability of amino acids in biogenic sediments and aqueous solutions at seafloor hydrothermal temperatures. Geochem J 43(5):331–341
Kelley DS, Baross JA, Delaney JR (2002) Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu Rev Earth Planet Sci 30(1):385–491. doi:10.1146/annurev.earth.30.091201.141331
Kobayashi K, Kohara M, Gamo T, Yanagawa H (1995) Formation and alteration of organic compounds in simulated submarine hydrothermal vent environments. In: Sakai H, Nozaki Y (eds) Bioorganic processes and the Ocean Flux in the Western Pacific. Terrapub, Tokyo, pp 532–535
Kobayashi K, Kohara M, Dokiya M, Yanagawa H (1997) Formation and stability of amino acids in simulated hydrothermal vent environments. Viva Origino 25:167–176
Kohara M, Gamo T, Yanagawa H, Kobayashi K (1997) Stability of amino acids in simulated hydrothermal vent environments. Chem Lett 10:1053–1054
Kurihara H, Takano Y, Kaneko T, Obayashi Y, Kobayashi K (2012) Stability of amino acids and related compounds in simulated submarine hydrothermal systems. Bull Chem Soc Jpn 85(5):624–630
Leach BE, Angelici RJ (1968) Metal-ion catalysis of the hydrolysis of some amino acid ester N, N-diacetic acids. J Am Chem Soc 90(10):2504–2508
Long DA, Truscott TG (1963) Peptide kinetics. Part 2.-Acid-catalyzed hydrolysis of glycyl-L-leucyl-glycine. Trans Faraday Soc 59:918–923. doi:10.1039 TF9635900918
Matsuno K (1997) A design principle of a flow reactor simulating prebiotic evolution. Viva Origino 25:191–204
Matsuno K, Swenson R (1999) Thermodynamics in the present progressive mode and its role in the context of the origin of life. Biosystems 51(1):53–61. doi:10.1016/S0303-2647(99)00013-1
Martin W, Baross J, Kelley D, Russell MJ (2008) Hydrothermal vents and the origin of life. Nat Rev Microbiol. doi:10.1038/nrmicro1991
Meng M, Stievano L, Lambert JF (2004) Adsorption and thermal condensation mechanisms of amino acids on oxide supports. 1. Glycine on silica. Langmuir 20(3):914–923. doi:10.1021/la035336b
Miller SL, Bada JL (1988) Submarine hot springs and the origin of life. Nature 334:609
Ogasawara H, Yoshida A, Imai E, Honda H, Hatori K, Matsuno K (2000) Synthesizing oligomers from monomeric nucleotides in simulated hydrothermal environments. Orig Life Evol Biosph 30(6):519–526
Ogata Y, Imai E, Honda H, Hatori K, Matsuno K (2000) Hydrothermal circulation of seawater through hot vents and contribution of interface chemistry to prebiotic synthesis. Orig Life Evol Biosph 30(6):527–537
Raju EV, Mathur HB (1968) The effect of inner orbital splitting on the thermodynamic properties of the transition metal complexes of serine and threonine. J Inorganice Nucl Chem 30(8):2181–2188. doi:10.1016/0022-1902(68)80215-5
Rode BM (1999) Peptides and the origin of life. Peptides 20(6):773–786
Shock E (1992) Chapter 5: chemical environment of submarine hydrothermal system. In: Holm H (ed) Marine hydrothermal system and the origin of life. Springer, Netherlands, pp 67–107
Wächtershäuser G (1990) Evolution of the first metabolic cycles. Proc Natl Acad Sci 87(1):200–204
Yanagawa H, Kobayashi Y, Egami F (1980) Characterization of marigranules and marisomes. Organized particles with Elastin-like structures. J Biochem 87:855–869
Acknowledgments
The authors would like to convey their gratitude to the following individuals for their efforts, guidance and motivation for this manuscript: Palash Kumar Sarkar; Pierre de Marcellus; Riddhideep Mandal, Christel Halliez, Yukinori Kawamoto, Rajdeep Gautam, Yuichi Kondo, Hironari Kurihara, Veeramani Krishnan and Holitiana Macha Randrianandraina
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandru, K., Imai, E., Kaneko, T. et al. Survivability and Abiotic Reactions of Selected Amino Acids in Different Hydrothermal System Simulators. Orig Life Evol Biosph 43, 99–108 (2013). https://doi.org/10.1007/s11084-013-9330-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-013-9330-9