Abstract
Purpose
In Saudi Arabia, owing to the abundant livestock, manures are a source of commercial humic amendments. Climate (hot weather plus scarcity of water sources) makes composting very difficult and compulsory to obtain these humic amendments without any previous composting step. A substantial fraction of manures, cellulosic in nature, are unextractable by alkaline solutions. Our aim is to verify whether a sequential combination of acid hydrolysis and KOH extraction could result in higher yields and better quality of the humic extract.
Methods
Acid hydrolysis was applied to manures either (1) in a single step, with HCl or H2SO4, or (2) in two steps (Saeman method). In both cases, acid hydrolysis was followed by extraction with hot KOH.
Results
Acid hydrolysis before KOH extraction increases total extracted organic matter. If acid hydrolysis is performed, the proportion of humic fractions in the KOH extract alone is strongly increased, reaching very high values (up to 80 % of C in the KOH extract after Saeman acid hydrolysis). Acid hydrolysis enhances some optical indicators of humification (E 4 E 6, aromaticity, E BTZ ratio) in the KOH extract. Nevertheless, because increasing acid concentration enhances carbohydrate extraction but decreases that of compounds other than carbohydrates, the overall result may be a drop in the proportion of humic fractions in the whole extracted matter (acid hydrolysate + KOH extract).
Conclusions
Acid hydrolysis improves yields of commercial humic extracts from manures. However, care must be taken to avoid a decrease in the richness of humic fractions in the obtained products.
Similar content being viewed by others
Introduction
Liquid humic amendments are used in many countries to enhance crop production. Though doubtful or even negative results may appear sometimes (for instance, Pinheiro et al. 2010; Verlinden et al. 2010), recent reviews state their general usefulness for crops under field conditions (Rose et al. 2014). The use of humic amendments, usually added to irrigation water, is almost compulsory in arid countries, where soils are often extremely poor in organic matter, which translates in a general way to a poor soil biochemistry.
At a commercial scale, humic amendments are most commonly obtained from fossil sources such as leonardite, but other sources such as peat, composted lignin-rich residues, or livestock residues (manures) may be envisaged. A consequence of this heterogeneity in the sources is the heterogeneity with regard to the physico-chemical characteristics of the commercial humic products. Such a heterogeneity is enhanced by the fact that the features of the extracted humic compounds depend on the concentration and the nature of the chemical extractive agent (Stevenson 1982; Tan 2003; Zaccone et al. 2007).
The Kingdom of Saudi Arabia (KSA) is not rich in peat or leonardite, but there is abundance of feedlot livestock (especially, cow and sheep), which makes the collection of manures in great amounts very easy—an essential condition if extraction at a commercial scale is to be applied. Livestock manure, available in impressive amounts in some countries, may be a source for commercial humic products comparable to fossil sources such as leonardite (Tan 2003).
Fresh manures do not contain true humic substances (HS), which appear during decomposition or as a result of composting. The compost-derived humic products seem to give the best results in the enhancement of plant production (Rose et al. 2014). Information about the processes associated with composting animal manures is abundant (Bernal et al. 2009); actually, the hormonal-like activity of humic extracts from composted manures has been known since the 1970s (Hernando et al. 1969a, b, 1977). In KSA, however, composting on a mass scale is extremely difficult owing to the hot climate and the scarcity of water resources. The problem is worsened by the very long times often needed to attain compost maturity, even for manures (Mondini et al. 1996; Som et al. 2009). Thus, obtaining these humic amendments without any previous composting is almost compulsory.
In a previous work (Alrefai et al. 2015), we performed extraction trials from uncomposted sheep and cow manures, using KOH solutions at a range of concentrations (0.25–2 M), at different temperatures (100–150 °C). The extracts produced in this way have properties similar to those of humic substances, but the yield was not too big and the amount of unextracted dung remained high. By solid-state 13C-NMR, we showed the dominance in the residue of O-alkyl C forms, usually attributed to polysaccharides. It is reasonable to assume that they are mostly cellulose and other cell wall polysaccharides. These forms, barely extractable by hot KOH, are prone to be hydrolyzed by acid. Therefore, an obvious strategy to drop the amount of unextracted residue is to perform an acid attack before the alkaline extraction.
Here, we show the results of our experiences of obtaining humic alkaline extracts from uncomposted manures, with or without a previous acid hydrolysis step. The aim of our work is not only to increase the total yield (or, in other words, to decrease the amount of unextracted residue), but also to verify whether the humic character of the obtained alkaline extracts is enhanced as a result of acid hydrolysis.Footnote 1
Materials and methods
Manures
Sheep and cow manures were obtained from farms near Riyadh. Manures were air dried for several days in the open air and then passed through a 2 mm sieve. Subsamples were finely ground for chemical analyses. Table 1 summarizes their most relevant properties.
Alkaline extraction and acid hydrolysis
A flow diagram of the applied fractionation method is given in Fig. 1. Essentially, we applied the classic alkaline fractionation method to obtain humic and fulvic acids, either after a previous acid hydrolysis or without any previous acid hydrolysis step. All procedures were performed in triplicate.
(a) Direct extraction with KOH Dried ground manures (500 mg) were extracted with 20 ml of 1N KOH, for 1 h at 105 °C, in sealed Pyrex tubes placed in a heating block. Alkaline extracts were recovered by centrifugation. Unextracted residues were washed with water; the washings were recovered also by centrifugation and combined with the alkaline extracts. Extracts were filtered through sintered glass filters, acidified to pH 7.5 and stored at 4 °C until analyses.
(b) Extraction with KOH after single-step acid hydrolysis Dried ground manures (500 mg) were hydrolyzed with 20 ml of HCl (0.1, 0.5 or 1N), or with 20 ml of H2SO4 (0.1, 0.5 or 1N), for 1 h at 105 °C in sealed Pyrex tubes placed in a heating block. The hydrolysates were recovered by centrifugation. Unhydrolyzed residues were washed with water; the washings were recovered also by centrifugation and combined with the hydrolysates. Hydrolysates were filtered through sintered glass filters. Unhydrolyzed residues were then extracted with 20 ml of 1N KOH, as explained above. The two consecutive liquid extracts (acid hydrolysate and KOH extract) were not combined; each one was studied separately. Both were stored at 4 °C until analysis.
(c) Extraction with KOH after two-step Saeman acid hydrolysis Saeman acid hydrolysis (Saeman et al. 1945) is the common method to hydrolyze cellulose and leave the so-called Klason lignin; it has ben also applied to the quantification of lignin in animal excreta (e.g., Kraus and Marlett 1990). Briefly, dried ground manures (500 mg) were hydrolyzed in a Pyrex tube, with 2 ml of 72 % H2SO4 at room temperature overnight in an end-over-end shaker. Water was then added to the tube to drop acid concentration to 1N and manures were hydrolyzed at 105 °C 1 h in a heating block. Recovery of hydrolysates and further extraction with KOH were done as in (b). Again, the acid hydrolysates and the KOH extracts were not combined, but studied separately.
None of these steps was performed under inert atmosphere (N2 or Ar). The reasons and implications of this choice are discussed further (see “Methodological constraints”). Both acidic and alkaline extracts were analyzed for carbon in a TOC analyzer. In all cases, the unextracted solid residue was recovered, dried, weighed and analyzed for carbon in a ThermoQuest CHN analyzer. From these, and taking into account the total C in the initial manure, the amount of C lost in the process was obtained:
Unaccounted C = Initial C − C in acid hydrolysate − C in the KOH extract.
Carbohydrates
In all acid hydrolysates and alkaline extracts, carbohydrates were quantified by the phenol–sulfuric method of Dubois et al. (1956), using glucose as standard. In the KOH extracts, to account for the possibility that a substantial part of carbohydrates were still in polysaccharide form, the aliquot to be analyzed was hydrolyzed previously with 1N H2SO4 (proportion 1:1 v/v) at 105 °C for 1 h. Carbohydrates were obtained as glucose equivalents, and its carbon was estimated by multiplying the obtained result by 0.4.
Fractionation of the hydrolysates and the KOH extracts
The KOH extracts were fractionated into humic acids, fulvic acids and non-humic compounds. Briefly, 50 ml of the extract was acidified to pH 1.0 with 2N H2SO4 and left to stand for 4 h. The insoluble fraction (humic acids: HA) was recovered by centrifugation and the soluble fraction (fulvic fraction) was saved. The humic acids were redissolved with 0.1N KOH, acidified again to pH 1.0 and centrifuged. The soluble fraction was saved and combined with the previous one. Humic acids were redissolved with a minimum amount of 0.1N KOH, its pH settled to 7.5, made up to 50 ml in a volumetric flask and stored at 4 °C.
The fulvic fraction (acid soluble) was fractionated through a column of Amberlite XAD-7 resin. The not retained, colorless material (non-humic fraction: NH) was recovered. The brown material, retained by the resin (fulvic acids: FA) was recovered by elution with NaOH (0.1N first and 1N thereafter), collected at the bottom of the column, acidified to pH 7.5 with 2N H2SO4, made up to 50 ml in a volumetric flask and stored at 4 °C.
Acid hydrolysates were also fractionated in this way, except for the first step (HA precipitation), because acid-insoluble compounds are by definition absent in them.
Both HA and FA (in the acid hydrolysate: FA I, and in the alkaline extract: FA II and HA II) were analyzed for carbon in a TOC analyzer. In the NH fractions (NH I and II), carbon was not analyzed directly, for they had become largely diluted during the fractionation. Their carbon contents were quantified by the difference: total alkaline extract − (HA II + FA II) in the alkaline extract; total hydrolysate − FA I in the acid hydrolysates.
From the C quantified in each fraction, we obtained the degree of humification (DH), according to Ciavatta et al. (1988):
where TEC is the total extracted carbon. This ratio measures how rich a given extract in humic fractions is and may be applied to the hydrolysate, the KOH extract or to the sum of both, i.e., the overall extracted matter. Ciavatta’s index has been preferred to other indicators, because all commercial humic products must declare (i.e., in the label) its relative richness in humic substances, which is considered by the farmers to be an indicator of the quality of the purchased product.
Optical properties
Optical properties are often used as indicators of the humification degree of humic substances, and they have been suggested as a criterion to classify them (Kumada 1987). To evaluate the apparent humification degree of the obtained fractions, three optical properties were studied:
-
(a)
The ratio between absorptions at 465 and 665 nm (E 4 E 6 ratio), in solutions of 150 µg C ml−1 and pH 7.5, as recommended by Chen et al. (1977).
-
(b)
In a second solution of 15 µg C ml−1 and pH 7.5, absorptions at 280, 253 and 203 nm were measured. The ratio between absorptions at 253 and 203 nm (Korshin et al. 1997) was then calculated (henceforth, E BTZ ratio). In addition to the E BTZ ratio, the E 253/E 220 ratio (Fuentes et al. 2006) was also applied to the obtained humic fractions. The latter is the ratio between absorptions at 253 and 220 nm, and it was proposed as an alternative to the former, because the absorption at 203 nm may be affected by the presence of nitrate, which absorbs also at this wavelength. However in our work, the original E BTZ ratio (253/203) gave more consistent results and allowed detecting more differences between the humic fractions depending on their origin (sheep or cow manure). Thus, only the results for the E BTZ ratio will be shown here.
Optical properties were studied in all the humic fractions: FA I, FA II and HA II. The non-humic fractions (NH I and NH II) were not included in this study. All absorptions were measured in 1-cm quartz cuvettes, in a T80 UV/vis spectrometer (PG Instruments). The E 4 E 6 ratio, a classical index of humification, has been largely considered an optical indicator of condensation of the humic molecules (Stevenson 1982). Chen et al. (1977), in a detailed study, showed that it is related to the apparent size of the molecule, but not to the concentration of condensed aromatic rings. The specific UV absorption at 280 nm (SUVA), normalized per 100 µg C ml−1 cm−1, is widely used as an indicator of abundance of aromatic C in the solution (Chin et al. 1994; Kalbitz et al. 2003). With regard to the absorptions at 253 and 203 nm, they are related to the electron-transfer bands of benzene and to the benzenoid band of benzene rings, respectively (Korshin et al. 1997; Fuentes et al. 2006).
As shown by Fuentes et al. (2006), all these indices increase with humification and under this point of view were applied in our experiment, i.e., as a criterion to detect increases or decreases in the apparent degree of humification in the humic fractions obtained.
Results
Carbon balance: extracted and lost
Table 2 summarizes the amounts of total C extracted either in the acid hydrolysis (with HCl, H2SO4 or by the Saeman hydrolysis) or in the subsequent alkaline extraction, or the sum of both. The yields obtained by direct alkaline extraction with KOH, without any previous hydrolysis step, are given also for comparison.
For both sheep and cow manure, acid hydrolysis increases the total extracted carbon. The highest yields are always obtained through the Saeman hydrolysis. Irrespective of the experimental condition, yields are much higher for sheep than for cow manure.
For a given acid (either HCl or H2SO4), increasing acid concentration results in an increased extraction of carbon in the acid hydrolysate, but a decrease of carbon in the subsequent alkaline extract (Fig. 2). It is noteworthy that for both sheep and cow manures, the maximum yield in the acid hydrolysate is obtained by the Saeman hydrolysis; but the yield of the KOH extraction after this hydrolysis is one of the lowest. Thus, the amount of C extracted in the acid hydrolysis and in the KOH extraction are inversely related (Table 2). As a result, increasing acid concentration from 0.1 to 1.0N does not always increase the total amount of extracted C. Actually in one case (Table 2: cow manure, hydrolysis with 1N H2SO4), the total extracted C does not significantly differ from that extracted with KOH without any previous acid hydrolysis.
Carbon losses in the process (acid hydrolysis plus/or KOH extraction) are small for sheep manure, but quite high for cow manure (Table 2). Losses by volatilization (due to decarboxylation reactions or to generation of volatile organic compounds) may partly explain the low yields obtained from cow manure and are always smaller than those obtained from sheep manure.
Extracted carbohydrates
Acid hydrolysis clearly increases carbohydrate extraction (Table 3). Whatever the conditions for acid hydrolysis, the amounts of extracted carbohydrates are much higher for sheep—than for cow manure. At high acid concentrations (1N), the effectiveness of HCl and H2SO4 seem similar, but at lower concentrations H2SO4 is more effective for polysaccharide hydrolysis. For both sheep and cow manure, carbohydrate extraction is highest by the Saeman hydrolysis method: the carbohydrates extracted from sheep manure by this procedure account for more than 40 % of the total initial C, and more than 30 % from cow manure.
Using 0.1N HCl for acid hydrolysis, the amount of carbohydrates extracted in the acid hydrolysate and in the alkaline extract is similar. In the rest of the cases, the acid hydrolysate accounts for most of the extracted carbohydrates. The amount of carbohydrates in the KOH extract is irrelevant.
Increasing the amount of carbohydrates released by acid hydrolysis does not automatically translate to a net increase in total extracted C (acid hydrolysate plus KOH extract). Even though acid hydrolysis improves substantially the extraction of carbohydrates, it does not improve (even decreases) the extraction of other compounds (Table 3). Hydrolysis with 0.1N HCl is the only exception to this rule.
Fractionation of acid hydrolysates and alkaline extracts
In absolute amounts (i.e., as unit of C extracted from unit of initial C in the extracted manure), increasing the concentration of acid in the previous acid hydrolysis results mainly in a net increase in NH I and, to a lesser amount, also FA I. In contrast, all fractions of the subsequent KOH extract tend to decrease in absolute amounts, leaving aside the increase in HA at the lowest acid concentration (Fig. 3).
Yields of all fractions are higher when extracted from sheep manure. Except for the FA I fraction, the kind of acid also affects the results. For extracting non-humic substances, hydrolysis with H2SO4 is more efficient than with HCl; in the subsequent KOH extraction, this results in lower amounts of non-humic substances (NH II) and fulvic acids (FA II). In contrast, yields of humic acids (HA II) are higher using sulfuric acid in the hydrolysis. Only when using the highest concentration (H2SO4 1N), a drop in the yield of humic acids is observed.
Saeman hydrolysis gives results outside the general trends. It yields very high amounts of non-humic substances in the hydrolysate (NH I) and also of humic acids in the KOH extract (HA II). In contrast, the yield of non-humic substances in the KOH extract (NH II) is remarkably low for sheep-derived manure. In the rest of the fractions, Saeman hydrolysis yields amounts similar to those obtained using the highest concentration of H2SO4 (1N).
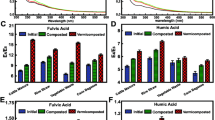
The overall result of the above-described behavior is a general decrease in the humic character of the total extracted carbon with acid concentration (Fig. 4, right graphs): the higher the acid concentration (for both HCl and H2SO4), the lower is the humification index. However, if only the KOH extract is considered (Fig. 4, middle graphs), the humification index is overall higher than for the whole C and tends to increase with acid concentration, except for the highest concentration of H2SO4 (1N).
Humification index (humic fractions/total C) in the acid hydrolysate (left), KOH extract (middle) and the sum of both (right). Points are averages of n = 3, and vertical bars show standard deviations. Data for Saeman hydrolysis have been plotted together with the other conditions, but out of scale, since in this procedure acid concentration is not constant
For both sheep and cow manures, the highest humification indices (both I and II) are obtained when acid hydrolysis is carried out following Saeman’ procedure. The humification index is particularly high for the KOH extract obtained from sheep manure: >80 % of the KOH-extracted C is found in the humic fractions (FA II and HA II).
Optical indicators of humification
E 4 E 6 ratios
In the FA I fraction obtained from cow manure, the E 4 E 6 ratio is not affected by acid concentration, both using HCl and H2SO4. However, this was the exception; in the rest of the studied fractions, the E 4 E 6 was largely affected by the acid used for hydrolysis and its concentration. The results were inconsistent, depending on the kind of manure (Fig. 5).
Using HCl for hydrolysis, increasing acid concentration increases the E 4 E 6 ratio of the studied fractions: the effect is clear for sheep manure and less for cow manure. In contrast, using H2SO4, the behavior of E 4 E 6 is largely inconsistent. In humic acids (HA II), the E 4 E 6 ratios increase with acid concentration. For fulvic acids, a two-phase behavior is observed: E 4 E 6 ratios increase for low acid concentrations, but drop again beyond a given threshold (0.5N for sheep-derived FA II, 0.1N for cow derived). In sheep-derived FA I obtained using H2SO4 for hydrolysis, increasing acid concentration consistently decreases E 4 E 6 ratios.
Aromaticity of humic fractions (absorption at 280 nm)
The aromaticity of fulvic acids in the hydrolysates (FA I) is the lowest of all humic fractions (Fig. 6). Aromaticity seems also little affected by the acid used (HCl or H2SO4) or its concentration and is very similar in sheep and cow manure-derived hydrolysates.
Specific UV absorption (SUVA) values, normalized as per solutions of 100 µg C per ml, measured in 1 cm quartz cuvettes. Points are averages of n = 3; vertical bars show standard deviations. Data for Saeman hydrolysis have been plotted together with the other conditions, but out of scale, since in this procedure acid concentration is not constant
The aromaticity of the humic fractions extracted in the subsequent KOH extraction, in contrast, is strongly affected by the previous acid hydrolysis. Aromaticities are usually higher if acid hydrolysis has been performed with H2SO4. If HCl is used, the aromaticity of both FA II and HA II consistently increases with the concentration of acid, for both sheep- and cow-derived manures. If H2SO4 is used, the aromaticity drops beyond a given threshold (either 0.1 or 0.5N), due probably to artifact generation. In HA II, the aromaticity increases with the concentration of acid; only for the highest H2SO4 concentration the trend seems to drop.
E BTZ ratio
In contrast to the E 4 E 6 ratio, or the absorption at 280 nm, the behavior of the E BTZ ratio in the humic fractions was homogeneous (Fig. 7). The ratio tended to increase with the concentration of the acid used in the hydrolysis step. The trend is very consistent; only in the FA I fraction obtained with HCl, an initial drop was observed from 0.1 to 0.5N. As a general trend, the E BTZ ratios are higher if acid hydrolysis has been performed with H2SO4. Increases are noteworthy (from about 0.22 to more than 0.40) in humic acids (HA II) when acid hydrolysis has been performed with H2SO4.
Discussion
Methodological constraints
In comparison to other studies on obtaining humic substances from organic sources, our work shows three peculiarities: (1) KOH is used throughout, instead of the more common NaOH; (2) extractions are performed under hot conditions and short times, instead of long extractions at room temperature; and (3) an inert atmosphere (of either N2 or Ar) was not applied in any of the extraction steps. All these peculiarities reflect the aim of applying extraction conditions as similar as possible to those expected when the extraction of humic products from manures will be applied at an industrial scale.
In scientific studies on humic substances, avoiding the generation of artifacts during the extraction is a main concern, and mild conditions are the common choice (extractions at room temperature and low NaOH concentrations, balanced by very long extraction times, often overnight): yield considerations are secondary. When humic amendments are to be obtained at a commercial scale, in contrast, yield considerations may never be secondary. Thus, harsh extraction conditions are usual. For instance, KOH is used at high concentrations (1 M or higher) to reach high extractions of humic matter, at the price of obtaining highly saline humic products, even though this is a minor problem because commercial humic products are largely diluted when applied to crops. With some exceptions (e.g., Garcia et al. 1994), it is usually observed that yields of extracted humic matter increase with temperature, at the price of shifts in the chemical characteristics of the extracted humic matter (Yefimov and Vasil’kova 1970; Cegarra et al. 1974; Garcia et al. 1994). Under these conditions, the obtained alkaline extracts may be partly artifactual, as well as their humic character.
We observed (Alrefai et al. 2015) that increasing extraction temperature of KOH concentration may mimic in the alkaline extracts the effect of composting, as deduced from their optical properties (Fuentes et al. 2006). The results of the current paper agree with this trend: most optical indicators of humification in the humic fractions (FA I and II, HA II) tend to increase with the concentration of acid used in the previous acid hydrolysis.
This principle may be also applied to the use of inert atmospheres (N2 or Ar) for humus extraction, a recommended practice in scientific studies (Swift 1996). There are detectable differences between the humic fractions extracted under inert atmosphere and those extracted in the presence of atmospheric O2 (Tan et al. 1991). However, applying an inert atmosphere for industrial-scale extraction of humus compounds would increase their cost notably, thus making them largely unattractive for farmers.
The unavoidable consequence of these harsh conditions (hot temperature, short time, lack of inert atmosphere) is the generation of artifacts during the hydrolysis and the further KOH extraction. However, we must accept that a substantial fraction of most commercial humic products are artefactual in nature. Artifact generation is the most reasonable explanation for most inconsistencies observed in our work about the behavior of humic fractions when increasing acid concentration in the hydrolysis step, such as erratic behaviors for E 4 E 6 ratios (Fig. 5) or sudden drops in aromaticity in FA II (Fig. 6).
Usefulness of acid hydrolysis
To obtain commercial humic products at an industrial scale, an acid hydrolysis step before the standard KOH extraction implies an added cost, which translates to the final product. Acid hydrolysis may be useful only if this added cost is balanced by yield increases, improvements of the obtained product—increased humic character—or both.
Yield increases are not always satisfactory, owing to the generation of artifacts: acid hydrolysis substantially improves the extraction of carbohydrates, but often it does not improve or even decrease that of other compounds (Table 3). Increasing acid concentration increases the yield of the acid hydrolysate, but decreases the yield of the further KOH extract, particularly for sheep manure (Table 2). The highest yields are obtained by the Saeman hydrolysis, but this procedure is longer and more complex than the rest and its applicability at a commercial scale is unclear.
As to the changes in the characteristics of the extracted matter, the proportion of humic fractions to total extracted matter (HD index) decreases with increasing acid concentration (Fig. 4). This is a relevant result, because commercial humic products are evaluated by their potential users (farmers) by their richness in humic or humic compounds, which in many countries must be stated in their labels, by law. Under such a point of view, acid hydrolysis may be a self-defeating practice. Nevertheless, it is not so simple. As shown in Fig. 6, the decrease in the humification index is largely due to the acid hydrolysate: on the contrary, in the KOH extract the HD may increase after acid hydrolysis. The apparent humification indices obtained in the KOH extract after Saeman’s hydrolysis are very high, particularly for sheep manure. On the other hand, most optical indicators used here show that increasing acid concentration may improve the apparent humic character of the fulvic and humic acids in the KOH extract: E 280, E BTZ and E 4 E 6 in FA II and HA II tend to increase with acid concentration, though not always in a consistent manner.
There are several ways by which acid hydrolysis may be useful for manufacturers. The first and most obvious method is to apply the conditions that apparently give the best results. Our results suggest that acid hydrolysis with a dilute acid solution (0.1N) is the best option: it results in increased total yield without any decrease in the humification ratio. The drawback of this option is that the small increase in total yield obtained under these conditions could not justify the cost of a previous acid hydrolysis. In such a case, other options could be considered:
(a) Taking separately the acid hydrolysate (rich in carbohydrates, poor in humic matter) and the KOH extract (poor in carbohydrates, rich in humic matter), rather than joining them in a single humic extract, is an option that may be considered. In this way, only the KOH extract, highly humic in character (Fig. 4), would be retained for use in agriculture as humic amendment. The challenge of this option should be to find a different and specific use for the acid hydrolysate: if this goal is attained, obtaining two different and consecutive products from a single substrate could be commercially interesting.
(b) Enhancing the humic character of the overall product (at least, the acid hydrolysate) by further chemical treatments. This option would take advantage of the available information about obtaining humic substances by purely chemical methods, a matter of study in soil science for many years (e.g., among many, Arfaioli et al. 2003; Cataldo 1998; Datta et al. 2001; Mathur and Schnitzer 1978; Wang and Huang 2000). The genesis of chemical analogs of humic substances relies often on secondary reactions between carbohydrates and amino acids in the presence of catalyzers; since carbohydrates and amino acids or peptides are expected to be the main constituents of the acid hydrolysate, such an option should be technically possible. Whether or not it could be feasible at an industrial scale from these hydrolysates should be the matter of future research.
End comment
In spite of the promising results obtained in this paper and in our previous one (Alrefai et al. 2015), a further step must be the study of the effects on plant growth of the obtained humic extracts and verifying the lack of deleterious effects on crops. The toxicity of manures usually disappears with composting. Since composting was avoided in our experiments, we must expect that the strong acid and/or alkaline treatments applied would have a similar effect, in the sense of provoking in the liquid extracts chemical changes strong enough to suppress any toxic effect. This compulsory step must be undertaken before translating our findings to a commercial or industrial scale.
Notes
We must state here that, since the manures used are fresh and uncomposted, in the alkaline extracts obtained from them strictly there will be no humic nor fulvic acids, but humic-like and fulvic-like acids. Nevertheless the terms ‘humic’ and ‘fulvic’ will be used throughout this paper, in order to avoid terminological complexities and make easier its reading.
References
Alrefai J, Rovira P, Alsewailem F, Abaalkheel I, Alsolami A, Albishi H, Alfantokh S (2015) Un-composted sheep and cow manures as a source of commercial humic amendments: an exploratory study. Commun Soil Sci Plant Anal 46:1137–1156. doi:10.1080/00103624.2015.1019084
Arfaioli P, Ugolini FC, Bosetto M, Corti G (2003) Synthesis of humic-like substances under sterile and non-sterile conditions in presence of quartz and volcanic ash. Commun Soil Sci Plant Anal 34:285–298
Bernal MP, Alburquerque JA, Moral R (2009) Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresr Technol 100:5444–5453
Cataldo F (1998) On the structure of macromolecules obtained by oxidative polymerization of polyhydroxyphenols and quinones. Polym Intern 46:263–268
Cegarra J, Reverte L, Lax A, Costa F (1974) Factores que influyen en la extracción y fraccionamiento de la materia orgánica del suelo. Anal Edafol Agrobiol 33:575–590
Chen Y, Senesi N, Schnitzer M (1977) Information provided on humic substances by E4/E6 ratios. Soil Sci Soc Am J 41:352–358
Chin YP, Aiken G, Loughlin EO (1994) Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ Sci Technol 28:1853–1858
Ciavatta C, Vittori Antisari L, Sequi P (1988) A first approach to the characterization of the presence of humified materials in organic fertilizers. Agrochimica 32:510–517
Datta A, Sanyal SK, Saha S (2001) A study on natural and synthetic humic acids and their complexing ability towards cadmium. Plant Soil 235:115–125
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–356
Fuentes M, González-Gaitano G, García-Mina JM (2006) The usefulness of UV-visible and fluorescence spectroscopies to study the chemical nature of humic substances from soils and composts. Org Geochem 37:1949–1959
Garcia D, Cegarra J, Roig A, Abad M (1994) Effects of the extraction temperature on the characteristics of a humic fertilizer obtained from lignite. Bioresr Technol 47:103–106
Hernando V, Sánchez Conde MP, Ortega BC (1969a) Acción del humato sódico sobre el desarrollo de diversas plantas (Action of sodium humate on the growth of several plants). Anal Edafol Agrobiol 28:791–809
Hernando V, Sánchez Conde MP, Ortega BC (1969b) Acción del ácido húmico sobre la planta de maíz cultivada en soluciones nutritivas equilibradas de concentración superior a la normal (Action of sodium humate on corn plants grown on nutrient solutions of concentration higher than usual). Anal Edafol Agrobiol 28:835–846
Hernando V, Ortega BC, Fortún C (1977) Estudio de la acción ejercida sobre la planta de maíz por dos tipos de ácido húmico [a study of the effect of two kinds of humic acid on corn plants]. In: Soil organic matter studies, vol 2, Rapport IAEA—SM—211/57. Pergamon Press: Oxford, pp 307–317
Kalbitz K, Schmerwitz J, Schwesig D, Matzner E (2003) Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 113:273–291
Korshin GV, Li CW, Benjamin MM (1997) Monitoring the properties of natural organic matter through UV spectroscopy: a consistent theory. Water Res 31:1787–1795
Kraus RJ, Marlett JA (1990) Measuring lignin in human and rat faeces. J Sci Food Agric 52:275–286
Kumada K (1987) Chemistry of soil organic matter. Elsevier, New York, p 241
Mathur SP, Schnitzer M (1978) A chemical and spectroscopic characterization of some synthetic analogues of humic acids. Soil Sci Soc Am J 42:591–596
Mondini C, Chiumenti R, da Borso F, Leita L, de Nobili M (1996) Changes during processing in the organic matter of composted and air-dried poultry manure. Bioresour Technol 55:243–249
Pinheiro GL, Silva CA, Furtini Neto AE (2010) Crescimento e nutriçâo de clone de eucalipto em resposta à aplicaçâo de concentraçôes de C-ácido húmico (Growth and nutrition of eucalyptus clone as affected by the application of C-humic acid doses). Rev Bras Ci Solo 34:1217–1229
Rose MT, Patti AF, Little KR, Brown AL, Jackson WR, Cavagnaro TR (2014) A meta-analysis and review of plant growth response to humic substances: practical implications for agriculture. Adv Agron 124:37–89
Saeman JF, Bubl JL, Harris EE (1945) Quantitative saccharification of wood and cellulose. Ind Eng Chem 17:35–37
Som MP, Lemée L, Amblès A (2009) Stability and maturity of a green waste and biowaste compost assessed on the basis of a molecular study using spectroscopy, thermal analysis, thermodesorption and thermochemolysis. Bioresour Technol 100:4404–4416
Song G, Novotny EH, Simpson A, Clapp CE, Hayes MHB (2008) Sequential exhaustive extraction of a Mollisol soil, and characterization of humic components, including humin, by solid and solution state NMR. Eur J Soil Sci 59:505–516
Stevenson FJ (1982) Humus chemistry. Genesis, composition, reactions. Wiley, New York
Swift RS (1996) Organic matter characterization. In: Sparks DL (ed), Methods of soil analysis, part 3, chemical methods. SSSA Book series no. 5, pp 1011–1070
Tan KH (2003) Humic matter in soil and the environment: principles and controversies. Marcel Dekker, New York
Tan KH, Lobartini JC, Himmelsbach DS, Asmussen LE (1991) Composition of humic acids extracted under air and nitrogen atmosphere. Commun Soil Sci Plant Anal 22:861–877
Verlinden G, Coussens T, de Vliegher A, Baert G, Haesaert G (2010) Effect of humic substances on nutrient uptake by herbage and on production and nutritive value of herbage from sown grass pastures. Grass Forage Sci 65:133–144
Wang MC, Huang PM (2000) Characteristics of pyrogallol-derived polymers formed by catalysis of oxides. Soil Sci 165:737–747
Yefimov VN, Vasil’kova MG (1970) Method of extracting humic substances from peat soils. Sov Soil Sci 2:335–345
Zaccone C, Cocozza C, D’Orazio V, Plaza C, Cheburkin A, Miano TM (2007) Influence of extractant on quality and trace elements content of peat humic acids. Talanta 73:820–830
Acknowledgments
This researched was financed by Grant Number 33-785, of the KACST (King Abdulaziz City for Science & Technology). The authors acknowledge also the CTFC (Forest Science Centre of Catalonia) for their technical support. Pere Rovira is a member of the Graccie-CONSOLIDER research network of the Spanish Ministry of Science and Innovation and also a member of the Consolidated Research Group CEMFOR (2014 SGR 1526), recognized by the Generalitat de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bellera, C., Abaalkheel, I., Rovira, P. et al. Obtaining commercial humic products from uncomposted manures: previous acid hydrolysis to enhance yields. Int J Recycl Org Waste Agricult 4, 219–231 (2015). https://doi.org/10.1007/s40093-015-0102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-015-0102-6