Abstract
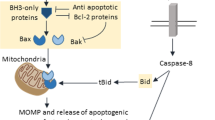
Release of cytochrome c from mitochondria triggers activation of caspase proteases and death of a cell by apoptosis. However, the mechanism and kinetics of cytochrome c release remain unknown. Here we study this event by using green fluorescent protein (GFP)-tagged cytochrome c, and find that the release of cytochrome-c–GFP always precedes exposure of phosphatidylserine and the loss of plasma-membrane integrity — characteristics of apoptotic cells. Once initiated, the release of cytochrome- c–GFP continues until all of the protein is released from all mitochondria in individual cells, within about 5 minutes, regardless of the type or strength of stimulus or the time elapsed since the stimulus was applied. Temperatures ranging from 24 °C to 37 °C do not change the duration of release, and nor does the addition of caspase inhibitors. Further, we find that the electron-transport chain can maintain the mitochondrial transmembrane potential even after cytochrome c has been released.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wolf, B. B. & Green, D. R. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem. 274, 20049–20052 (1999).
Slee, E. A. et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144, 281– 292 (1999).
Nicholson, D. W. & Thornberry, N. A. Caspases: killer proteases. Trends Biochem. Sci. 22, 299–306 (1997).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Zou, H., Li, Y., Liu, X. & Wang, X. An APAF-1/cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9 . J. Biol. Chem. 274, 11549– 11556 (1999).
Zou, H., Henzel, W. J., Liu, X., Lutschg, A. & Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3 . Cell 90, 405–413 (1997).
Cecconi, F., Alvarez-Bolado, G., Meyer, B. I., Roth, K. A. & Gruss, P. Apaf 1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94, 727–737 (1998).
Hu, Y., Benedict, M. A., Ding, L. & Nunez, G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase- 9 activation and apoptosis. EMBO J. 18, 3586–3595 (1999).
Srinivasula, S. M., Ahmad, M., Fernandes-Alnemri, T. & Alnemri, E. S. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell 1, 949–957 ( 1998).
Kennedy, S. G., Kandel, E. S., Cross, T. K. & Hay, N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 19, 5800–5810 (1999).
Narita, M. et al. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria . Proc. Natl Acad. Sci. USA 95, 14681– 14686 (1998).
Wolter, K. G. et al. Movement of Bax from the cytosol to mitochondria during apoptosis . J. Cell Biol. 139, 1281– 1292 (1997).
Finucane, D. M., Bossy-Wetzel, E., Waterhouse, N. J., Cotter, T. G. & Green, D. R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 274, 2225– 2233 (1999).
Eskes, R. et al. Bax-induced cytochrome c release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J. Cell Biol. 143, 217– 224 (1998).
Luo, X., Budihardjo, I., Zou, H., Slaughter, C. & Wang, X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94, 481– 490 (1998).
Desagher, S. et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144, 891–901 (1999).
Basanez, G. et al. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl Acad. Sci. USA 96, 5492–5497 ( 1999).
Marchetti, P. et al. Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 184, 1155 –1160 (1996).
Heiskanen, K. M., Bhat, M. B., Wang, H. W., Ma, J. & Nieminen, A. L. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC 6 cells. J. Biol. Chem. 274, 5654–5658 (1999).
Heiden, M. G., Chandel, N. S., Schumacker, P. T. & Thompson, C. B. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 3, 159–167 (1999).
Susin, S. A. et al. Mitochondrial release of caspase-2 and -9 during the apoptotic process. J. Exp. Med. 189, 381– 394 (1999).
Susin, S. A. et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441– 446 (1999).
Green, D. R. & Reed, J. C. Mitochondria and apoptosis. Science 281, 1309–1312 ( 1998).
Marzo, I. et al. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J. Exp. Med. 187, 1261–1271 ( 1998).
Bossy-Wetzel, E., Newmeyer, D. D. & Green, D. R. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17, 37–49 (1998).
Single, B., Leist, M. & Nicotera, P. Simultaneous release of adenylate kinase and cytochrome c in cell death. Cell Death Differ. 5, 1001–1003 (1998).
Kohler, C. et al. Release of adenylate kinase 2 from the mitochondrial intermembrane space during apoptosis. FEBS Lett. 447, 10–12 (1999).
Kluck, R. M. et al. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell Biol. 147, 809–822 (1999).
Martin, S. J. et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182 , 1545–1556 (1995).
Vermes, I., Haanen, C., Steffens-Nakken, H. & Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 184, 39–51 (1995).
Srinivasan, A. et al. Bcl-xL functions downstream of caspase-8 to inhibit Fas- and tumor necrosis factor receptor 1-induced apoptosis of MCF7 breast carcinoma cells. J. Biol. Chem. 273, 4523– 4529 (1998).
Matsuyama, S., Xu, Q., Velours, J. & Reed, J. C. The mitochondrial F0F1-ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells. Mol. Cell 1, 327–336 ( 1998).
Saleh, A., Srinivasula, S. M., Acharya, S., Fishel, R. & Alnemri, E. S. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J. Biol. Chem. 274, 17941–17945 (1999).
Eguchi, Y., Shimizu, S. & Tsujimoto, Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 57, 1835 –1840 (1997).
Leist, M., Single, B., Castoldi, A. F., Kuhnle, S. & Nicotera, P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis . J. Exp. Med. 185, 1481– 1486 (1997).
Kroemer, G., Dallaporta, B. & Resche-Rigon, M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60, 619 –642 (1998).
Acknowledgements
We thank C. Ware, M. Schuler, R. Kluck, P. Tailor, M. Pinkoski and D. Newmeyer for assistance. This work was supported by grants CA69381 and A140646 from the US NIH. J.C.G. was supported by training grant CA09345 from the US NCI.
Correspondence and requests for materials should be addressed to D.R.G.
Supplementary information is available on Nature Cell Biology’s World-Wide Web site (http://www.nature.com/ncb).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Movie 1
Individual mitochondria release cytochrome-c-GFP at different times within a 6-min period. An animated time-lapse of HeLa cells releasing cytochrome-c-GFP after UV radiation. The animated sequence contains six frames sand loops ten times. The time after UV exposure is displayed in the bottom left corner while the punctate/diffuse index is in the right corner. The white circle shows the area used to calculate the punctate/diffuse index. (MOV 186 kb)
Movie 2
A time-lapse of a single human cell (HeLa) undergoing programmed cell death (apoptosis) after exposure to the cytokine tumour necrosis factor. This cell expresses cytochrome c (a molecule implicated in apoptosis signalling) fused to the green fluorescent protein (GFP). After treatment, cytochrome c-GFP (green) moves from the mitochondria to the cytosol. The cell then rounds, blebs, and externalises phosphatidylserine, a phospholipid membrane component and phagocytosis signal identified by association with annexin V (red). Finally a DNA dye (blue) stains the nucleus following the collapse of plasma membrane integrity. The interval between each frame is ten minutes. (MOV 442 kb)
Rights and permissions
About this article
Cite this article
Goldstein, J., Waterhouse, N., Juin, P. et al. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2, 156–162 (2000). https://doi.org/10.1038/35004029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35004029
This article is cited by
-
PGAM5 exacerbates acute renal injury by initiating mitochondria-dependent apoptosis by facilitating mitochondrial cytochrome c release
Acta Pharmacologica Sinica (2024)
-
BAX activation in mouse retinal ganglion cells occurs in two temporally and mechanistically distinct steps
Molecular Neurodegeneration (2023)
-
Sub-lethal signals in the mitochondrial apoptosis apparatus: pernicious by-product or physiological event?
Cell Death & Differentiation (2023)
-
Dynamic assessment of the relationship between oxidative stress and apoptotic pathway in embryonic fibroblast cells exposed to glycidamide: possible protective role of hesperidin
Environmental Science and Pollution Research (2023)
-
Modeling oxidative injury response in human kidney organoids
Stem Cell Research & Therapy (2022)