Abstract
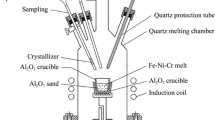
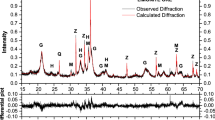
Fe–Ni–Cr–Co–P alloys were exposed to 17.3% CO2–H2 gas mixtures to investigate the oxidation of minor elements in metallic alloys in the early solar system. Reaction temperatures varied between 700 and 1000 °C. Gas-phase equilibrium was attained at 800, 900, and 1000 °C, yielding H2–H2O–CO–CO2 gas mixtures. Experiments at 700 and 750 °C did not achieve gas-phase equilibrium and were performed in H2–CO2 gas mixtures. Reaction timescales varied from 1 to 742 h. The experimental samples were characterized using optical microscopy, electron microprobe analysis, wavelength-dispersive-spectroscopy X-ray elemental mapping, and X-ray diffraction. In all experiments Cr experiences internal oxidation to produce inclusions of chromite (FeCr2O4) and eskolaite (Cr2O3) and surface layers of Cr-bearing magnetite [(Fe,Cr)3O4]. At 900 and 1000 °C, P is lost from the alloy via diffusion and sublimation from the metal surface. Analysis of P zoning profiles in the remnant metal cores allows for the determination of the P diffusion coefficient in the bulk metal, which is constant, and the internally oxidized layer, which is shown to vary linearly with distance from the metal surface. At 800 and 900 °C, P oxidizes to form a surface layer of graftonite [Fe3(PO4)2] while at 700 and 750 °C P forms inclusions of the phosphide-mineral schreibersite [(Fe,Ni)3P].







Similar content being viewed by others
References
D. S. Lauretta, D. T. Kremser, and B. Fegley, Icarus 122, 288 (1996).
D. S. Lauretta, K. Lodders, and B. Fegley, Science 277, 358 (1997).
D. S. Lauretta, K. Lodders, and B. Fegley, Meteoritics & Planetary Science 33, 821 (1998).
B. Fegley Jr., Space Science Reviews 92, 177 (2000).
B. Zanda, D. M. Bourot, C. Perron, and R. H. Hewins, Science 265, 1846 (1994).
D. S. Lauretta, P. R. Buseck, and T. J. Zega, Geochimica et Cosmichimica Acta 65, 1337 (2001).
D. S. Lauretta and P. R. Buseck, Meteoritics & Planetary Science 38, 59 (2003).
Y. Hong and B. Fegley Jr., Meteoritics and Planetary Science 33, 1101 (1998).
D. S. Lauretta, Oxidation of Metals 64, 1 (2005).
J. Megusar and G. H. Meier, Metallurgical Transactions A 7A, 1133 (1976).
J. Crank, The Mathematics of Diffusion (Oxford University Press, New York, 1975).
A. D. Le Claire and G. Neumann, in Numerical Data and Functional Relationship in Science and Technology. Landolt-Bornstein, New Series, Group III, ed. H. Mehrer, Vol. 26 (Springer Verlag, Berlin, 1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lauretta, D.S., Schmidt, B.E. Oxidation of Minor Elements from an Iron–Nickel–Chromium–Cobalt–Phosphorus Alloy in 17.3% CO2–H2 Gas Mixtures at 700–1000 °C. Oxid Met 71, 219–235 (2009). https://doi.org/10.1007/s11085-009-9140-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-009-9140-7