Ebola
| Ebola | |
|---|---|
| Specialty | Infectious diseases |
Ebola virus disease (EVD) or Ebola hemorrhagic fever (EHF) is the human disease which may be caused by any of four of the five known ebola viruses. These four viruses are: Bundibugyo virus (BDBV), Ebola virus (EBOV), Sudan virus (SUDV), and Taï Forest virus (TAFV, formerly and more commonly Côte d'Ivoire Ebola virus (Ivory Coast Ebolavirus, CIEBOV)). EVD is a viral hemorrhagic fever (VHF), and is clinically nearly indistinguishable from Marburg virus disease (MVD).
The name comes from the Ebola River in the Democratic Republic of the Congo, where it was first found.
Classification
The genera Ebolavirus and Marburgvirus were originally classified as the species of the now-obsolete Filovirus genus. In March 1998, the Vertebrate Virus Subcommittee proposed in the International Committee on Taxonomy of Viruses (ICTV) to change the Filovirus genus to the Filoviridae family with two specific genera: Ebola-like viruses and Marburg-like viruses. This proposal was implemented in Washington, DC on April 2001 and in Paris on July 2002. In 2000, another proposal was made in Washington, DC, to change the "-like viruses" to "-virus" resulting in today's Ebolavirus and Marburgvirus.[1]

Rates of genetic change are 100 times slower than influenza A in humans, but on the same magnitude as those of hepatitis B. Extrapolating backwards using these rates indicates that Ebolavirus and Marburgvirus diverged several thousand years ago.[2] However, paleoviruses (genomic fossils) of filoviruses (Filoviridae) found in mammals indicate that the family itself is at least tens of millions of years old.[3] Viral fossils that are closely related to ebolaviruses have been found in the genome of the Chinese hamster.[4]
The five characterised Ebola species are:
- Zaire ebolavirus (ZEBOV)
- Also known simply as the Zaire virus, ZEBOV has the highest case-fatality rate of the ebolaviruses, up to 90% in some epidemics, with an average case fatality rate of approximately 83% over 27 years. There have been more outbreaks of Zaire ebolavirus than of any other species. The first outbreak occurred on 26 August 1976 in Yambuku.[5] The first recorded case was Mabalo Lokela, a 44‑year-old schoolteacher. The symptoms resembled malaria, and subsequent patients received quinine. Transmission has been attributed to reuse of unsterilized needles and close personal contact.
- Sudan ebolavirus (SEBOV)
- Like the Zaire virus, SEBOV emerged in 1976; it was at first assumed to be identical with the Zaire species.[6] SEBOV is believed to have broken out first among cotton factory workers in Nzara, Sudan, with the first case reported as a worker exposed to a potential natural reservoir. The virus was not found in any of the local animals and insects that were tested in response. The carrier is still unknown. The lack of barrier nursing (or "bedside isolation") facilitated the spread of the disease. The most recent outbreak occurred in May, 2004. Twenty confirmed cases were reported in Yambio County, Sudan, with five deaths resulting. The average fatality rates for SEBOV were 54% in 1976, 68% in 1979, and 53% in 2000 and 2001.
- Reston ebolavirus (REBOV)
- Discovered during an outbreak of simian hemorrhagic fever virus (SHFV) in crab-eating macaques from Hazleton Laboratories (now Covance) in 1989. Since the initial outbreak in Reston, Virginia, it has since been found in non-human primates in Pennsylvania, Texas and Siena, Italy. In each case, the affected animals had been imported from a facility in the Philippines,[7] where the virus has also infected pigs.[8] Despite its status as a Level‑4 organism and its apparent pathogenicity in monkeys, REBOV did not cause disease in exposed human laboratory workers.[9]
- Côte d'Ivoire ebolavirus (CIEBOV)
- Also referred to as Taï Forest ebolavirus and by the English place name, "Ivory Coast", it was first discovered among chimpanzees from the Taï Forest in Côte d'Ivoire, Africa, in 1994. Necropsies showed blood within the heart to be brown; no obvious marks were seen on the organs; and one necropsy showed lungs filled with blood. Studies of tissues taken from the chimpanzees showed results similar to human cases during the 1976 Ebola outbreaks in Zaire and Sudan. As more dead chimpanzees were discovered, many tested positive for Ebola using molecular techniques. The source of the virus was believed to be the meat of infected Western Red Colobus monkeys, upon which the chimpanzees preyed. One of the scientists performing the necropsies on the infected chimpanzees contracted Ebola. She developed symptoms similar to those of dengue fever approximately a week after the necropsy, and was transported to Switzerland for treatment. She was discharged from the hospital after two weeks and had fully recovered six weeks after the infection.[10]
- Bundibugyo ebolavirus (BEBOV)
- On 24 November 2007, the Uganda Ministry of Health confirmed an outbreak of Ebolavirus in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the CDC, the World Health Organization confirmed the presence of the new species. On 20 February 2008, the Uganda Ministry officially announced the end of the epidemic in Bundibugyo, with the last infected person discharged on 8 January 2008.[11] An epidemiological study conducted by WHO and Uganda Ministry of Health scientists determined there were 116 confirmed and probable cases of the new Ebola species, and that the outbreak had a mortality rate of 34% (39 deaths). In 2012, there was an outbreak of Bundibugyo ebolavirus in a northeastern province of the Democratic Republic of the Congo. There were 15 confirmed cases and 10 fatalities.[12]
Signs and symptoms
This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: references need tidying. (September 2012) |
Ebola Virus Disease begins with a sudden onset of an influenza-like stage characterized by general malaise, fever with chills, arthralgia, myalgia, and chest pain. Nausea is accompanied by abdominal pain, diarrhea, and vomiting. Respiratory tract involvement is characterized by pharyngitis with sore throat, cough, dyspnea, and hiccups. The central nervous system is affected as judged by the development of severe headaches, agitation, confusion, fatigue, depression, seizures, and sometimes coma.
Cutaneous presentation may include: maculopapular rash, petechiae, purpura, ecchymoses, and hematomas (especially around needle injection sites). Development of hemorrhagic symptoms is generally indicative of a negative prognosis. However, contrary to popular belief, hemorrhage does not lead to hypovolemia and is not the cause of death (total blood loss is low except during labor). Instead, death occurs due to multiple organ dysfunction syndrome (MODS) due to fluid redistribution, hypotension, disseminated intravascular coagulation, and focal tissue necroses.
The mean incubation period, best calculated currently for EVD outbreaks due to EBOV infection, is 12.7 days (standard deviation = 4.3 days), but can be as long as 25 days.[13]
Hemorrhage
All patients show some extent of coagulopathy and impaired circulatory system symptomology.[14] Bleeding from mucous membranes and puncture sites is reported in 40–50% of cases,[15] while maculopapular rashes are evident in approximately 50% of cases.[14] Sources of bleeds include hematemesis, hemoptysis, melena, and aforementioned bleeding from mucous membranes (gastroinestinal tract, nose, vagina and gingiva). Diffuse bleeding, however, is rare, and is usually exclusive to the gastrointestinal tract.[14][16]
Causes
EVD is caused by four of five viruses classified in the genus Ebolavirus, family Filoviridae, order Mononegavirales: Bundibugyo virus (BDBV), Ebola virus (EBOV), Sudan virus (SUDV), and Taï Forest virus (TAFV). The fifth virus, Reston virus (RESTV), is thought to be apathogenic for humans and therefore not discussed here.
| Species name | Virus name (Abbreviation) |
|---|---|
| Bundibugyo ebolavirus (accepted)[17] | Bundibugyo virus (BDBV; previously BEBOV) |
| Sudan ebolavirus | Sudan virus (SUDV; previously SEBOV) |
| Taï Forest ebolavirus | Taï Forest virus (TAFV; previously CIEBOV) |
| Zaire ebolavirus* | Ebola virus (EBOV; previously ZEBOV) |
Table legend: "*" denotes the type species and "accepted" refers to a taxon that has been accepted by the Executive Committee of the ICTV but that has yet to be ratified.
Risk factors
Between 1976 and 1998, from 30,000 mammals, birds, reptiles, amphibians, and arthropods sampled from outbreak regions, no ebolavirus was detected apart from some genetic traces found in six rodents (Mus setulosus and Praomys) and one shrew (Sylvisorex ollula) collected from the Central African Republic.[18][19] Traces of EBOV were detected in the carcasses of gorillas and chimpanzees during outbreaks in 2001 and 2003, which later became the source of human infections. However, the high lethality from infection in these species makes them unlikely as a natural reservoir.[18]
Plants, arthropods, and birds have also been considered as possible reservoirs; however, bats are considered the most likely candidate.[20] Bats were known to reside in the cotton factory in which the index cases for the 1976 and 1979 outbreaks were employed, and they have also been implicated in Marburg virus infections in 1975 and 1980.[18] Of 24 plant species and 19 vertebrate species experimentally inoculated with EBOV, only bats became infected.[21] The absence of clinical signs in these bats is characteristic of a reservoir species. In a 2002–2003 survey of 1,030 animals which included 679 bats from Gabon and the Republic of the Congo, 13 fruit bats were found to contain EBOV RNA fragments.[22] As of 2005, three types of fruit bats (Hypsignathus monstrosus, Epomops franqueti, and Myonycteris torquata) have been identified as being in contact with EBOV. They are now suspected to represent the EBOV reservoir hosts.[23]
The existence of integrated genes of filoviruses in some genomes of small rodents, insectivorous bats, shrews, tenrecs, and marsupials indicates a history of infection with filoviruses in these groups as well.[3] However, it has to be stressed that infectious ebolaviruses have not yet been isolated from any nonhuman animal.
Bats drop partially eaten fruits and pulp, then terrestrial mammals such as gorillas and duikers feed on these fallen fruits. This chain of events forms a possible indirect means of transmission from the natural host to animal populations, which have led to research towards viral shedding in the saliva of bats. Fruit production, animal behavior, and other factors vary at different times and places which may trigger outbreaks among animal populations.[24] Transmission between natural reservoirs and humans are rare, and outbreaks are usually traceable to a single index case where an individual has handled the carcass of gorilla, chimpanzee, or duiker.[25] The virus then spreads person-to-person, especially within families, hospitals, and during some mortuary rituals where contact among individuals becomes more likely.[26]
The virus has been confirmed to be transmitted through body fluids. Transmission through oral exposure and through conjunctiva exposure is likely[27] and has been confirmed in non-human primates.[28] Filoviruses are not naturally transmitted by aerosol. They are, however, highly infectious as breathable 0.8–1.2 micrometre droplets in laboratory conditions;[29] because of this potential route of infection, these viruses have been classified as Category A biological weapons.[30]
All epidemics of Ebola have occurred in sub-optimal hospital conditions, where practices of basic hygiene and sanitation are often either luxuries or unknown to caretakers and where disposable needles and autoclaves are unavailable or too expensive. In modern hospitals with disposable needles and knowledge of basic hygiene and barrier nursing techniques, Ebola has never spread on a large scale. In isolated settings such as a quarantined hospital or a remote village, most victims are infected shortly after the first case of infection is present. The quick onset of symptoms from the time the disease becomes contagious in an individual makes it easy to identify sick individuals and limits an individual's ability to spread the disease by traveling. Because bodies of the deceased are still infectious, some doctors had to take measures to properly dispose of dead bodies in a safe manner despite local traditional burial rituals.[31]
Virology
Genome

Like all mononegaviruses, ebolavirions contain linear nonsegmented, single-stranded, non-infectious RNA genomes of negative polarity that possesses inverse-complementary 3' and 5' termini, do not possess a 5' cap, are not polyadenylated, and are not covalently linked to a protein.[32] Ebolavirus genomes are approximately 19 kilobase pairs long and contain seven genes in the order 3'-UTR-NP-VP35-VP40-GP-VP30-VP24-L-5'-UTR.[33] The genomes of the five different ebolaviruses (BDBV, EBOV, RESTV, SUDV, and TAFV) differ in sequence and the number and location of gene overlaps.
Structure
Like all filoviruses, ebolavirions are filamentous particles that may appear in the shape of a shepherd's crook or in the shape of a "U" or a "6", and they may be coiled, toroid, or branched.[33] Ebolavirions are generally 80 nm in width, but vary somewhat in length. In general, the median particle length of ebolaviruses ranges from 974–1,086 nm (in contrast to marburgvirions, whose median particle length was measured to be 795–828 nm), but particles as long as 14,000 nm have been detected in tissue culture.[34] Ebolavirions consist of seven structural proteins. At the center is the helical ribonucleocapsid, which consists of the genomic RNA wrapped around a polymer of nucleoproteins (NP). Associated with the ribonucleoprotein is the RNA-dependent RNA polymerase (L) with the polymerase cofactor (VP35) and a transcription activator (VP30). The ribonucleoprotein is embedded in a matrix, formed by the major (VP40) and minor (VP24) matrix proteins. These particles are surrounded by a lipid membrane derived from the host cell membrane. The membrane anchors a glycoprotein (GP1,2) that projects 7 to 10 nm spikes away from its surface. While nearly identical to marburgvirions in structure, ebolavirions are antigenically distinct.
Entry
Niemann–Pick C1 (NPC1) appears to be essential for Ebola infection. Two independent studies reported in the same issue of Nature (journal) showed that Ebola virus cell entry and replication requires the cholesterol transporter protein NPC1.[35][36] When cells from Niemann Pick Type C1 patients (who have a mutated form of NPC1) were exposed to Ebola virus in the laboratory, the cells survived and appeared immune to the virus, further indicating that Ebola relies on NPC1 to enter cells. This might imply that genetic mutations in the NPC1 gene in humans could make some people resistant to one of the deadliest known viruses affecting humans. The same studies described similar results with Ebola's cousin in the filovirus group, Marburg virus, showing that it too needs NPC1 to enter cells.[35][36] Furthemore, NPC1 was shown to be critical to filovirus entry because it mediates infection by binding directly to the viral envelope glycoprotein.[36] A later study confirmed the findings that NPC1 is a critical filovirus receptor that mediates infection by binding directly to the viral envelope glycoprotein and that the second lysosomal domain of NPC1 mediates this binding.[37]
In one of the original studies, a small molecule was shown to inhibit Ebola virus infection by preventing the virus glycoprotein from binding to NPC1.[36][38] In the other study, mice that were heterozygous for NPC1 were shown to be protected from lethal challenge with mouse adapted Ebola virus.[35] Together, these studies suggest NPC1 may be potential therapeutic target for an Ebola anti-viral drug.
Replication
The ebolavirus life cycle begins with virion attachment to specific cell-surface receptors, followed by fusion of the virion envelope with cellular membranes and the concomitant release of the virus nucleocapsid into the cytosol. The viral RNA polymerase, encoded by the L gene, partially uncoats the nucleocapsid and transcribes the genes into positive-stranded mRNAs, which are then translated into structural and nonstructural proteins. Ebolavirus RNA polymerase (L) binds to a single promoter located at the 3' end of the genome. Transcription either terminates after a gene or continues to the next gene downstream. This means that genes close to the 3' end of the genome are transcribed in the greatest abundance, whereas those toward the 5' end are least likely to be transcribed. The gene order is therefore a simple but effective form of transcriptional regulation. The most abundant protein produced is the nucleoprotein, whose concentration in the cell determines when L switches from gene transcription to genome replication. Replication results in full-length, positive-stranded antigenomes that are, in turn, transcribed into negative-stranded virus progeny genome copy. Newly synthesized structural proteins and genomes self-assemble and accumulate near the inside of the cell membrane. Virions bud off from the cell, gaining their envelopes from the cellular membrane they bud from. The mature progeny particles then infect other cells to repeat the cycle.[39]
Pathophysiology
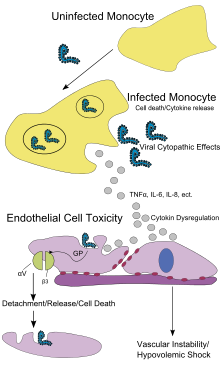
Endothelial cells, mononuclear phagocytes, and hepatocytes are the main targets of infection. After infection, in a secreted glycoprotein (sGP) the Ebola virus glycoprotein (GP) is synthesized. Ebola replication overwhelms protein synthesis of infected cells and host immune defenses. The GP forms a trimeric complex, which binds the virus to the endothelial cells lining the interior surface of blood vessels. The sGP forms a dimeric protein which interferes with the signaling of neutrophils, a type of white blood cell, which allows the virus to evade the immune system by inhibiting early steps of neutrophil activation. These white blood cells also serve as carriers to transport the virus throughout the entire body to places such as the lymph nodes, liver, lungs, and spleen.[40] The presence of viral particles and cell damage resulting from budding causes the release of cytokines (specifically TNF-α, IL-6, IL-8, etc.), which are the signaling molecules for fever and inflammation. The cytopathic effect, from infection in the endothelial cells, results in a loss of vascular integrity. This loss in vascular integrity is furthered with synthesis of GP, which reduces specific integrins responsible for cell adhesion to the inter-cellular structure, and damage to the liver, which leads to coagulopathy.[41]
Diagnosis
EVD is clinically indistinguishable from Marburg virus disease (MVD), and it can also easily be confused with many other diseases prevalent in Equatorial Africa, such as other viral hemorrhagic fevers, falciparum malaria, typhoid fever, shigellosis, rickettsial diseases such as typhus, cholera, gram-negative septicemia, borreliosis such as relapsing fever or EHEC enteritis. Other infectious diseases that ought to be included in the differential diagnosis include leptospirosis, scrub typhus, plague, Q fever, candidiasis, histoplasmosis, trypanosomiasis, visceral leishmaniasis, hemorrhagic smallpox, measles, and fulminant viral hepatitis. Non-infectious diseases that can be confused with EVD are acute promyelocytic leukemia, hemolytic uremic syndrome, snake envenomation, clotting factor deficiencies/platelet disorders, thrombotic thrombocytopenic purpura, hereditary hemorrhagic telangiectasia, Kawasaki disease, and even warfarin intoxication.[42][43][44][45]
The most important indicator that may lead to the suspicion of EVD at clinical examination is the medical history of the patient, in particular the travel and occupational history (which countries were visited?) and the patient's exposure to wildlife (exposure to bats, bat excrement, nonhuman primates?). EVD can be confirmed by isolation of ebolaviruses from or by detection of ebolavirus antigen or genomic or subgenomic RNAs in patient blood or serum samples during the acute phase of EVD. Ebolavirus isolation is usually performed by inoculation of grivet kidney epithelial Vero E6 or MA-104 cell cultures or by inoculation of human adrenal carcinoma SW-13 cells, all of which react to infection with characteristic cytopathic effects.[46][47] Filovirions can easily be visualized and identified in cell culture by electron microscopy due to their unique filamentous shapes, but electron microscopy cannot differentiate the various filoviruses alone despite some overall length differences.[34] Immunofluorescence assays are used to confirm ebolavirus presence in cell cultures. During an outbreak, virus isolation and electron microscopy are most often not feasible options. The most common diagnostic methods are therefore RT-PCR[48][49][50][51][52][53][54] in conjunction with antigen-capture ELISA[55][56][57][58][59] which can be performed in field or mobile hospitals and laboratories. Indirect immunofluorescence assays (IFAs) are not used for diagnosis of EVD in the field anymore.
Prevention

Ebola viruses are highly infectious as well as contagious.
As an outbreak of ebola progresses, bodily fluids from diarrhea, vomiting, and bleeding represent a hazard. Due to lack of proper equipment and hygienic practices, large-scale epidemics occur mostly in poor, isolated areas without modern hospitals or well-educated medical staff. Many areas where the infectious reservoir exists have just these characteristics. In such environments, all that can be done is to immediately cease all needle-sharing or use without adequate sterilization procedures, isolate patients, and observe strict barrier nursing procedures with the use of a medical-rated disposable face mask, gloves, goggles, and a gown at all times, strictly enforced for all medical personnel and visitors.[60] The aim of all of these techniques is to avoid any person’s contact with the blood or secretions of any patient, including those who are deceased.[61]
Vaccines have successfully protected nonhuman primates; however, the six months needed to complete immunization made it impractical in an epidemic. To resolve this, in 2003, a vaccine using an adenoviral (ADV) vector carrying the Ebola spike protein was tested on crab-eating macaques. The monkeys were challenged with the virus 28 days later, and remained resistant.[62] In 2005, a vaccine based on attenuated recombinant vesicular stomatitis virus (VSV) vector carrying either the Ebola glycoprotein or Marburg glycoprotein successfully protected nonhuman primates,[63] opening clinical trials in humans.[64] By October, the study completed the first human trial; giving three vaccinations over three months showing capability of safely inducing an immune response. Individuals were followed for a year, and, in 2006, a study testing a faster-acting, single-shot vaccine began. This study was completed in 2008.[65] The next step is to try the vaccine on a strain of Ebola that is closer to the one that infects humans.[citation needed]
There are currently no Food and Drug Administration-approved vaccines for the prevention of EVD. Many candidate vaccines have been developed and tested in various animal models.[66][67][68] Of those, the most promising ones are DNA vaccines[69] or are based on adenoviruses,[62] vesicular stomatitis Indiana virus (VSIV)[70][71][72] or filovirus-like particles (VLPs)[73] as all of these candidates could protect nonhuman primates from ebolavirus-induced disease. DNA vaccines, adenovirus-based vaccines, and VSIV-based vaccines have entered clinical trials.[64][65][74][75]
Contrary to popular belief, ebolaviruses are not transmitted by aerosol during natural EVD outbreaks. Due to the absence of an approved vaccine, prevention of EVD therefore relies predominantly on behavior modification, proper personal protective equipment, and sterilization/disinfection.
On 6 December 2011, the development of a successful vaccine against Ebola for mice was reported. Unlike the predecessors, it can be freeze-dried and thus stored for long periods in wait for an outbreak. The research is reported in Proceedings of National Academy of Sciences.[76]
In endemic zones
The natural maintenance hosts of ebolaviruses remain to be identified. This means that primary infection cannot necessarily be prevented in nature. The avoidance of EVD risk factors, such as contact with nonhuman primates or bats, is highly recommended, but may not be possible for inhabitants of tropical forests or people dependent on nonhuman primates as a food source.
During outbreaks
Since ebola viruses do not spread via aerosol, the most straightforward prevention method during EVD outbreaks is to avoid direct (skin-to-skin) contact with patients, their excretions and body fluids, or possibly contaminated materials and utensils. Patients should be isolated and medical staff should be trained and apply strict barrier nursing techniques (disposable face mask, gloves, goggles, and a gown at all times). Traditional burial rituals, especially those requiring embalming of bodies, should be discouraged or modified, ideally with the help of local traditional healers.[60]
In the laboratory
Ebolaviruses are World Health Organization Risk Group 4 Pathogens, requiring Biosafety Level 4-equivalent containment. Laboratory researchers have to be properly trained in BSL-4 practices and wear proper personal protective equipment.
Treatment

There is currently no FDA-approved ebolavirus-specific therapy for EVD. Treatment is primarily supportive in nature and includes minimizing invasive procedures, balancing fluids and electrolytes to counter dehydration, administration of anticoagulants early in infection to prevent or control disseminated intravascular coagulation, administration of procoagulants late in infection to control hemorrhaging, maintaining oxygen levels, pain management, and administration of antibiotics or antimycotics to treat secondary infections.[77][78][79] Hyperimmune equine immunoglobulin raised against EBOV has been used in Russia to treat a laboratory worker who accidentally infected herself with EBOV—but the patient died anyway.[80] Experimentally, recombinant vesicular stomatitis Indiana virus (VSIV) expressing the glycoprotein of EBOV or SUDV has been used successfully in nonhuman primate models as post-exposure prophylaxis.[81][82] Such a recombinant post-exposure vaccine was also used to treat a German researcher who accidentally pricked herself with a possibly EBOV-contaminated needle. Treatment might have been successful as she survived. However, actual EBOV infection could never be demonstrated without a doubt.[83] Novel, very promising, experimental therapeutic regimens rely on antisense technology. Both small interfering RNAs (siRNAs) and phosphorodiamidate morpholino oligomers (PMOs) targeting the EBOV genome could prevent disease in nonhuman primates.[84][85]
During an outbreak in the Democratic Republic of the Congo in 1995, seven of eight patients who received blood transfusions from convalescent individuals survived.[86] However, this potential treatment is considered controversial.[6]
Prognosis
Prognosis is generally poor (average case-fatality rate of all EVD outbreaks to date = 68%). If a patient survives, recovery may be prompt and complete, or protracted with sequelae, such as orchitis, arthralgia, myalgia, desquamation or alopecia. Ocular manifestations, such as photophobia, hyperlacrimation, iritis, iridocyclitis, choroiditis and blindness have also been described. Importantly, EBOV and SUDV are known to be able to persist in the sperm of some survivors, which could give rise to secondary infections and disease via sexual intercourse.[87][88][89][90][91]
Epidemiology
Outbreaks of EVD have mainly been restricted to Africa. The virus often consumes the population. Governments and individuals quickly respond to quarantine the area while the lack of roads and transportation helps to contain the outbreak.[7] EVD was first described after almost simultaneous viral hemorrhagic fever outbreaks occurred in Zaire and Sudan in 1976.[92][93] EVD is believed to occur after an ebolavirus is transmitted to a human index case via contact with an infected animal host. Human-to-human transmission occurs via direct contact with blood or bodily fluids from an infected person (including embalming of a deceased victim) or by contact with contaminated medical equipment such as needles. In the past, explosive nosocomial transmission has occurred in underequipped African hospitals due to the reuse of needles and lack of implementation of universal precautions. Aerosol transmission has not been observed during natural EVD outbreaks. The potential for widespread EVD epidemics is considered low due to the high case-fatality rate, the rapidity of demise of patients, and the often remote areas where infections occur.
| Year | Virus | Geographic location | Human cases/deaths (case-fatality rate |
|---|---|---|---|
| 1976 | SEBOV | Juba, Maridi, Nzara, and Tembura, Sudan | 284/151 (53%) |
| 1976 | EBOV | Yambuku, Zaire | 318/280 (88%) |
| 1977 | EBOV | Bonduni, Zaire | 1/1 (100%) |
| 1979 | SUDV | Nzara, Sudan | 34/22 (65%) |
| 1988 | EBOV | Porton Down, United Kingdom | 1/0 (0%) [laboratory accident] |
| 1994 | TAFV | Taï National Park, Côte d'Ivoire | 1/0 (0%) |
| 1994–1995 | EBOV | Woleu-Ntem and Ogooué-Ivindo Provinces, Gabon | 52/32 (62%) |
| 1995 | EBOV | Kikwit, Zaire | 317/245 (77%) |
| 1996 | EBOV | Mayibout 2, Gabon | 31/21 (68%) |
| 1996 | EBOV | Sergiyev Posad, Russia | 1/1 (100%) [laboratory accident] |
| 1996–1997 | EBOV | Ogooué-Ivindo Province, Gabon; Cuvette-Ouest Department, Republic of the Congo | 62/46 (74%) |
| 2000–2001 | SUDV | Gulu, Mbarara, and Masindi Districts, Uganda | 425/224 (53%) |
| 2001–2002 | EBOV | Ogooué-Ivindo Province, Gabon; Cuvette-Ouest Department, Republic of the Congo | 124/97 (78%) |
| 2002 | EBOV | Ogooué-Ivindo Province, Gabon; Cuvette-Ouest Department, Republic of the Congo | 11/10 (91%) |
| 2002–2003 | EBOV | Cuvette-Ouest Department, Republic of the Congo; Ogooué-Ivindo Province, Gabon | 143/128 (90%) |
| 2003–2004 | EBOV | Cuvette-Ouest Department, Republic of the Congo | 35/29 (83%) |
| 2004 | EBOV | Koltsovo, Russia | 1/1 (100%) [laboratory accident] |
| 2004 | SUDV | Yambio County, Sudan | 17/7 (41%) |
| 2005 | EBOV | Cuvette-Ouest Department, Republic of the Congo | 11/9 (82%) |
| 2007 | EBOV | Kasai Occidental Province, Democratic Republic of the Congo | 264/186 (71%) |
| 2007–2008 | BDBV | Bundibugyo District, Uganda | 116/39 (34%) |
| 2008–2009 | EBOV | Kasai Occidental Province, Democratic Republic of the Congo | 32/15 (47%) |
| 2011 | SUDV | Luweero District, Uganda | 1/1 (100%) |
| 2012 | SUDV | Kibaale District, Western Uganda | 24/17 (71%) |
| 2012 | BDBV | Orientale Province, Democratic Republic of the Congo | 62/34 (54%) |
While investigating an outbreak of Simian hemorrhagic fever virus (SHFV) in November 1989, an electron microscopist from USAMRIID discovered filoviruses similar in appearance to Ebola in tissue samples taken from crab-eating macaque imported from the Philippines to Hazleton Laboratories Reston, Virginia.[94]
Blood samples were taken from 178 animal handlers during the incident.[95] Of those, six animal handlers eventually seroconverted. When the handlers failed to become ill, the CDC concluded that the virus had a very low pathogenicity to humans.[96]
Because of the virus's high mortality, it is a potential agent for biological warfare.[97]
Given the lethal nature of Ebola, and since no approved vaccine or treatment is available, it is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention. It has the potential to be weaponized for use in biological warfare.[98]
The BBC reports in a study that frequent outbreaks of Ebola may have resulted in the deaths of 5,000 gorillas.[99]
Recent cases
As of 30 August 2007, 103 people (100 adults and three children) were infected by a suspected hemorrhagic fever outbreak in the village of Kampungu, Democratic Republic of the Congo. The outbreak started after the funerals of two village chiefs, and 217 people in four villages fell ill. The World Health Organization sent a team to take blood samples for analysis and confirmed that many of the cases are the result of Ebolavirus.[100][101] The Congo's last major Ebola epidemic killed 245 people in 1995 in Kikwit, about 200 miles (320 km) from the source of the August 2007 outbreak.[102]
On 30 November 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization confirmed the presence of a new species of Ebolavirus which is now tentatively named Bundibugyo.[103] The epidemic came to an official end on 20 February 2008. While it lasted, 149 cases of this new strain were reported, and 37 of those led to deaths.
An International Symposium to explore the environment and filovirus, cell system and filovirus interaction, and filovirus treatment and prevention was held at Centre Culturel Français, Libreville, Gabon, during March 2008.[104] The virus appeared in southern Kasai Occidental on 27 November 2008,[105] and blood and stool samples were sent to laboratories in Gabon and South Africa for identification.
On 25 December 2008, a mysterious disease that had killed 11 and infected 21 people in southern Democratic Republic of Congo was identified as the Ebola virus.[106] Doctors Without Borders reported 11 deaths as of 29 December 2008 in the Western Kasai province of the Democratic Republic of Congo, stating that a further 24 cases were being treated. In January 2009, Angola closed down part of its border with DRC to prevent the spread of the outbreak.[107]
On 12 March 2009, an unidentified 45-year-old scientist from Germany accidentally pricked her finger with a needle used to inject Ebola into lab mice. She was given an experimental vaccine never before used on humans. Since the peak period for an outbreak during the 21-day Ebola incubation period has passed as of 2 April 2009, she has been declared healthy and safe. It remains unclear whether or not she was ever actually infected with the virus.[108]
In May 2011, a 12-year-old girl in Uganda died from Ebola (Sudan subspecies). No further cases were recorded.[109]
In December 2011, an unidentified woman presented at a Nairobi hospital with "Ebola-like symptoms" and subsequently died. The pathogen has yet to be identified.[110]
2012 outbreaks
In July 2012, the Ugandan Health Ministry confirmed 13 deaths due to an outbreak of the Ebola-Sudan variant[111] in the Kibaale District.[112] As of 28 July 2012, 14 out of 20 (70% mortality rate) had died in Kibaale.[113] On July 30, Stephen Byaruhanga, a health official in Kibaale District, said the Ebola outbreak has spread from one remote village to several villages.[114]
The World Health Organization's global and alert response network reported on August 3 that the suspected case count had risen to 53, including 16 deaths. Of these cases, five were confirmed by UVRI as Ebola cases. There have been no confirmed cases outside of Kibaale District except for a patient who was medically evacuated to Kampala District and has since died. WHO and CDC support is on the ground in Uganda supporting the government response. There have been no confirmed cases outside of Uganda.[115] Included among the populations confirmed to be affected are prisoners in Kabbale prison. One of the inmates suspected of infection escaped from medical isolation on the same day.[116] Dr. Joaquim Saweka, the WHO representative to Uganda, also reported that the outbreak was then under control and that everyone known to have had contact with a known Ebola patient is now in isolation.[117]
On 8 August 2012, the Ugandan Ministry of Health has recorded 23 probable and confirmed cases, including 16 deaths. Ten cases were confirmed by the Uganda Virus Research Institute as Ebola. 185 people who came into contact with probable and confirmed Ebola cases are being followed up during the incubation period of 21 days.[118]
On 17 August 2012, the Ministry of Health of the Democratic Republic of the Congo reported an outbreak of the Ebola-Bundibugyo variant[119] in the eastern region.[120] By August 21, the WHO reported a total of 15 cases and 10 fatalities.[121] There is no evidence to date that this outbreak is connected to the Ugandan outbreak.[122] By 13 September 2012, the World Health Organisation revealed that the virus had claimed 32 lives and that the probable cause of the outbreak was tainted bush-meat hunted by local villagers around the towns of Isiro and Viadana.[123]
History

Ebolavirus first emerged in 1976 in outbreaks of Ebola hemorrhagic fever in Zaire and Sudan.[124] The strain of Ebola that broke out in Zaire has one of the highest case fatality rates of any human pathogenic virus, roughly 90%.[125]
The Philippines and the United States had no previous cases of infection, and upon further isolation it was concluded to be another strain of Ebola or a new filovirus of Asian origin, and named Reston ebolavirus (REBOV) after the location of the incident.
In other animals
Outbreaks of EVD among human populations generally result from handling infected wild animal carcasses. Declines in animal populations generally precede outbreaks among human populations. Since 2003, such declines have been monitored through surveillance of animal populations with the aim of predicting and preventing EVD outbreaks in humans.[126] Recovered carcasses from gorillas contain multiple Ebola virus strains, which suggest multiple introductions of the virus. Bodies decompose quickly and carcasses are not infectious after three to four days. Contact between gorilla groups is rare, suggesting transmission among gorilla groups is unlikely, and that outbreaks result from transmission between viral reservoir and animal populations.[127]
Outbreaks of EVD may have been responsible for an 88% decline in tracking indices of observed chimpanzee populations in 420 square kilometer Lossi Sanctuary between 2002–2003.[127] Transmission among chimpanzees through meat consumption constitutes a significant 5.2 (1.3–21.1 with 95% confidence) relative risk factor, while contact between individuals, such as touching dead bodies and grooming, do not.[128]
Domestic animals
Ebola virus can be transmitted to dogs and pigs.[129] While dogs may be asymptomatic pigs tend to develop clinical disease.
Recent research
In late 2012, Canadian scientists discovered that the deadliest form of the virus could be transmitted by air between species.[130] They managed to prove that the virus was transmitted from pigs to monkeys without any direct contact between them, leading to fears that airborne transmission could be contributing to the wider spread of the disease in parts of Africa. Evidence was also found that pigs might be one of the reservoir hosts for the virus; the fruit bat has long been considered as the reservoir.[130]
References
- ^ Büchen-Osmond, Cornelia (2006-04-25). "ICTVdB Virus Description – 01.025.0.02. Ebolavirus". International Committee on Taxonomy of Viruses. Retrieved 2009-06-02.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9254917, please use {{cite journal}} with
|pmid=9254917instead. - ^ a b c Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20569424, please use {{cite journal}} with
|pmid= 20569424instead. - ^ Taylor, D. J. (2011). "Evolutionary maintenance of filovirus-like genes in bat genomes". BMC Evolutionary Biology. 11. doi:10.1186/1471-2148-11-336.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Isaacson, M; Sureau, P; Courteille, G; Pattyn, SR;. "Clinical Aspects of Ebola Virus Disease at the Ngaliema Hospital, Kinshasa, Zaire, 1976". Retrieved 2009-07-08.
{{cite journal}}: Cite journal requires|journal=(help); Invalid|ref=harv(help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21084112, please use {{cite journal}} with
|pmid=21084112instead. - ^ a b Special Pathogens Branch CDC (2008-01-14). "Known Cases and Outbreaks of Ebola Hemorrhagic Fever". Center for Disease Control and Prevention. Retrieved 2008-08-02.
- ^ McNeil Jr, Donald G. (2009-01-24). "Pig-to-Human Ebola Case Suspected in Philippines". New York Times. Retrieved 2009-01-26.
- ^ McCormick & Fisher-Hoch 1999, p. 300
- ^ Waterman, Tara (1999). Ebola Cote D'Ivoire Outbreaks. Stanford University. Retrieved 2009-05-30.
- ^ "End of Ebola outbreak in Uganda" (Press release). World Health Organization. 2008-02-20.
- ^ Wamala, J; Lukwago, L; Malimbo, M; Nguku, P; Yoti, Z; Musenero, M; Amone, J; Mbabazi, W; Nanyunja, M; Zaramba, S; Opio, A; Lutwama, J; Talisuna, A; Okware, I; (2010). "Ebola Hemorrhagic Fever Associated with Novel Virus Strain, Uganda, 2007–2008". Emerging Infectious Disease. 16 (7). Retrieved 2010-06-24.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Eichner, Martin; Dowell, Scott F.; Firese, Nina (2011). "Incubation Period of Ebola Hemorrhagic Virus Subtype Zaire OH AND BRETT". Osong Public Health and Research Perspectives. 2 (1): 3–7. doi:10.1016/j.phrp.2011.04.001.
- ^ a b c Hoenen, Thomas (2006). "Ebola virus: unravelling pathogenesis to combat a deadly disease". Trends in Molecular Medicine. 12 (5): 206–215.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ "Medscape: Ebola Virus, Clinical Presentation". Retrieved 2012-07-30.
- ^ Fisher-Hoch, S.P. (1985). "Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola)". J. Infect. Dis. 152: 887–894.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kuhn, Jens H.; Becker, Stephan; Ebihara, Hideki; Geisbert, Thomas W.; Johnson, Karl M.; Kawaoka, Yoshihiro; Lipkin, W. Ian; Negredo, Ana I; Netesov, Sergey V. (2010). "Proposal for a revised taxonomy of the family Filoviridae: Classification, names of taxa and viruses, and virus abbreviations". Archives of Virology. 155 (12): 2083–103. doi:10.1007/s00705-010-0814-x. PMC 3074192. PMID 21046175.
- ^ a b c Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.micinf.2005.04.006, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.micinf.2005.04.006instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S1286-4579(99)00242-7, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S1286-4579(99)00242-7instead. - ^ "Fruit bats may carry Ebola virus". BBC News. 2005-12-11. Retrieved 2008-02-25.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8969248, please use {{cite journal}} with
|pmid=8969248instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/438575a, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/438575ainstead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17940947, please use {{cite journal}} with
|pmid=17940947instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17848072, please use {{cite journal}} with
|pmid=17848072instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15078595, please use {{cite journal}} with
|pmid=15078595instead. - ^ Questions and Answers about Ebola Hemorrhagic Fever. Centers for Disease Control and Prevention. 2009-03-25. Retrieved 2009-05-31.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8551825, please use {{cite journal}} with
|pmid=8551825instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8712894, please use {{cite journal}} with
|pmid=8712894instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7547435, please use {{cite journal}} with
|pmid=7547435instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15588056, please use {{cite journal}} with
|pmid=15588056instead. - ^ Harden, Blaine (2001-02-18). "Dr. Matthew's Passion". New York Times Magazine. Retrieved 2008-02-25.
- ^ Pringle, C. R. (2005), "Order Mononegavirales", in Fauquet, C. M.; Mayo, M. A.; Maniloff, J.; Desselberger, U.; Ball, L. A. (eds.), Virus Taxonomy—Eighth Report of the International Committee on Taxonomy of Viruses, San Diego, USA: Elsevier/Academic Press, pp. 609–614, ISBN 0-12-370200-3
- ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7118520, please use {{cite journal}} with
|pmid=7118520instead. - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8837880, please use {{cite journal}} with
|pmid=8837880instead. - ^ a b c Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR (2011). "Ebola virus entry requires the cholesterol transporter Niemann-Pick C1". Nature. 477 (7364): 340–3. doi:10.1038/nature10348. PMC 3175325. PMID 21866103.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J (2011). "Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection". Nature. 477 (7364): 344–8. doi:10.1038/nature10380. PMC 3230319. PMID 21866101.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Miller EH, Obernosterer G, Raaben M, Herbert AS, Deffieu MS, Krishnan A, Ndungo E, Sandesara RG, Carette JE, Kuehne AI, Ruthel G, Pfeffer SR, Dye JM, Whelan SP, Brummelkamp TR, Chandran K (2012). "Ebola virus entry requires the host-programmed recognition of an intracellular receptor". EMBO Journal. 31 (8): 1947–60. doi:10.1038/emboj.2012.53. PMC 3343336. PMID 22395071.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Flemming A (2011). "Achilles heel of Ebola viral entry". Nat Rev Drug Discov. 10 (10): 731. doi:10.1038/nrd3568. PMID 21959282.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Feldmann, H.; Geisbert, T. W.; Jahrling, P. B.; Klenk, H.-D.; Netesov, S. V.; Peters, C. J.; Sanchez, A.; Swanepoel, R.; Volchkov, V. E. (2005), "Family Filoviridae", in Fauquet, C. M.; Mayo, M. A.; Maniloff, J.; Desselberger, U.; Ball, L. A. (eds.), Virus Taxonomy—Eighth Report of the International Committee on Taxonomy of Viruses, San Diego, USA: Elsevier/Academic Press, pp. 645–653, ISBN 0-12-370200-3
- ^ Smith, Tara (2005). Ebola (Deadly Diseases and Epidemics). Chelsea House Publications. ISBN 0-7910-8505-8.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1128/JVI.77.18.9733-9737.2003, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1128/JVI.77.18.9733-9737.2003instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 2665013, please use {{cite journal}} with
|pmid=2665013instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 565951, please use {{cite journal}} with
|pmid=565951instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16267962, please use {{cite journal}} with
|pmid=16267962instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9612907, please use {{cite journal}} with
|pmid=9612907instead. - ^ Ksiazek, Thomas G. (1991). "Laboratory diagnosis of filovirus infections in nonhuman primates". Lab Animal. 20 (7): 34–6.
- ^ van der Groen, G.; Webb, P.; Johnson, K.; Lange, J.; Lindsay, H.; Eliot, L. (1978), "Growth of Lassa and Ebola viruses in different cell lines", in Pattyn, S. R. (ed.), Ebola Virus Haemorrhagic Fever, Amsterdam, Netherlands: Elsevier/North-Holland Biomedical Press, pp. 255–260, ISBN 0-444-80060-3
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9988180, please use {{cite journal}} with
|pmid=9988180instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10686031, please use {{cite journal}} with
|pmid=10686031instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12089242, please use {{cite journal}} with
|pmid=12089242instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11682540, please use {{cite journal}} with
|pmid=11682540instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15047846, please use {{cite journal}} with
|pmid=15047846instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15072761, please use {{cite journal}} with
|pmid=15072761instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17079496, please use {{cite journal}} with
|pmid=17079496instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 1572982, please use {{cite journal}} with
|pmid=1572982instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11526161, please use {{cite journal}} with
|pmid=11526161instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12821193, please use {{cite journal}} with
|pmid=12821193instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 14593476, please use {{cite journal}} with
|pmid=14593476instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16857271, please use {{cite journal}} with
|pmid=16857271instead. - ^ a b Centers for Disease Control and Prevention and World Health Organization (1998). Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (PDF). Atlanta, Georgia, USA: Centers for Disease Control and Prevention. Retrieved 2013-02-08.
- ^ Center for Disease Control, Special Pathogens Branch. Questions and Answers about Ebola Hemorrhagic Fever (PDF). Atlanta, Georgia, USA: Center for Disease Control.
- ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nature01876, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nature01876instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nm1258, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nm1258instead. - ^ a b Oplinger, Anne A. (2003-11-18). NIAID Ebola vaccine enters human trial. Bio-Medicine.
- ^ a b "Ebola/Marburg Vaccine Development" (Press release). National Institute of Allergy and Infectious Diseases. 2008-09-15.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8017061, please use {{cite journal}} with
|pmid=8017061instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 6108462, please use {{cite journal}} with
|pmid=6108462instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11996686, please use {{cite journal}} with
|pmid=11996686instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9427604, please use {{cite journal}} with
|pmid=9427604instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18930776, please use {{cite journal}} with
|pmid=18930776instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19043556, please use {{cite journal}} with
|pmid=19043556instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19386702, please use {{cite journal}} with
|pmid=19386702instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17940980, please use {{cite journal}} with
|pmid=17940980instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16988008, please use {{cite journal}} with
|pmid=16988008instead. - ^ Bush, L. (2005), "Crucell and NIH sign Ebola vaccine manufacturing contract", Pharmaceutical Technology, 29: 28
- ^ Phoolcharoen, W.; Dye, J. M.; Kilbourne, J.; Piensook, K.; Pratt, W. D.; Arntzen, C. J.; Chen, Q.; Mason, H. S.; Herbst-Kralovetz, M. M. (2011). "A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge". Proceedings of the National Academy of Sciences. 108 (51): 20695–20700. Bibcode:2011PNAS..10820695P. doi:10.1073/pnas.1117715108. [1]
Related news article:- Jennifer Carpenter (6 December 2011). "Vaccine developed against Ebola". BBC News.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17940941, please use {{cite journal}} with
|pmid=17940941instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16483416, please use {{cite journal}} with
|pmid=16483416instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15460155, please use {{cite journal}} with
|pmid=15460155instead. - ^ Akinfeeva, L. A.; Aksyonova, O. I.; Vasilevich, I. V.; Ginko, Z. I.; Zarkov, K. A.; Zubavichene, L. R.; Kuzovlev, O. P.; Kuzubov, V. I.; Ryabchikova, Ye. I. (2005). "A case of Ebola hemorrhagic fever". Infektsionnye Bolezni (3): 85–88.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17238284, please use {{cite journal}} with
|pmid=17238284instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18385248, please use {{cite journal}} with
|pmid=18385248instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19307268, please use {{cite journal}} with
|pmid=19307268instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20511019, please use {{cite journal}} with
|pmid=20511019instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20729866, please use {{cite journal}} with
|pmid=20729866instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9988160, please use {{cite journal}} with
|pmid=9988160instead. - ^ Bowen, E. T. W.; Lloyd, G.; Platt, G.; McArdell, L. B.; Webb, P. A.; Simpson, D. I. H. (1978), "Vitrological Studies on a Case of Ebola Virus Infection in Man and Monkeys", in Pattyn, S. R. (ed.), Ebola Virus Haemorrhagic Fever, Amsterdam, Netherlands: Elsevier/North-Holland Biomedical Press, pp. 95–102, ISBN 0-444-80060-3
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9988158, please use {{cite journal}} with
|pmid=9988158instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9988159, please use {{cite journal}} with
|pmid=9988159instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9988181, please use {{cite journal}} with
|pmid=9988181instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9988162, please use {{cite journal}} with
|pmid=9988162instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 307455, please use {{cite journal}} with
|pmid=307455instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 307456, please use {{cite journal}} with
|pmid=307456instead. - ^ McCormick & Fisher-Hoch 1999, pp. 277–279
- ^ Waterman, Tara (1999). Ebola Reston Outbreaks. Stanford University. Retrieved 2008-08-02.
- ^ McCormick & Fisher-Hoch 1999, pp. 298–299
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15207310, please use {{cite journal}} with
|pmid=15207310instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1001/jama.287.18.2391, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1001/jama.287.18.2391instead. - ^ Ebola 'kills over 5,000 gorillas'. BBC. 2006-12-08. Retrieved 2009-05-31.
- ^ "Ebola Outbreak Confirmed in Congo". NewScientist.com. 2007-09-11. Retrieved 2008-02-25.
- ^ Ebola outbreak in Congo. CDC news. 2007-09-12. Retrieved 2009-05-31.
- ^ "Mystery DR Congo fever kills 100". BBC News. 2007-08-31. Retrieved 2008-02-25.
- ^ "Uganda: Deadly Ebola Outbreak Confirmed – UN". UN News Service. 2007-11-30. Retrieved 2008-02-25.
- ^ The IV International Symposium on Filoviruses. l'Institut de recherche pour le développement (IRD). Retrieved 2009-0-31.
{{cite book}}: Check date values in:|accessdate=(help) - ^ World Health Organization (2008-12-27). RD Congo: Fièvre hémorragique à virus Ebola au Kasaï Occidental, Rapport de situation No 1 des 26 & 27 décembre 2008 (in French). Relief Web. Retrieved 2009-06-02.
- ^ Ebola epidemic kills nine in central DR Congo: report. Agence France-Presse. 2008-12-25. Retrieved 2009-05-30.
- ^ Ebola alert shuts Angolan border. BBC. 2009-01-06. Retrieved 2009-05-31.
- ^ Eddyn, Melissan (2009-03-27). "Scientist Injects Self With Ebola". Associated Press. Retrieved 2009-05-02.
- ^ Malone, Barry (2011-06-17). "Uganda says Ebola outbreak is over". Reuters. Retrieved 2011-07-06.
- ^ Bernard Momanyi and Catherina Karongo (December 22, 2011). "Panic as woman dies of Ebola-like symptoms at KNH". Capital FM News. Kenya.
- ^ Norway (2012-08-24). "Congo (DRC): Bushmeat blamed for Ebola outbreak - Norwegian Council for Africa". Afrika.no. Retrieved 2013-04-15.
- ^ "Outbreak of Ebola in Uganda kills 13". BBC News. July 28, 2012.
- ^ "Officials: Uganda Ebola outbreak kills 14 - Health | NBC News". MSNBC. 2012-07-28. Retrieved 2013-04-15.
- ^ "Ebola Outbreak Spreads". Associated Press – The Express. July 31, 2012.
{{cite news}}:|access-date=requires|url=(help) - ^ "WHO | Ebola in Uganda – update". Who.int. 2012-08-03. Retrieved 2013-04-15.
- ^ http://www.cnn.com/2012/08/03/health/uganda-ebola-virus/index.html
- ^ "WHO: Ebola Outbreak in Uganda is Under Control : US/World". Medical Daily. 2012-08-03. Retrieved 2013-04-15.
- ^ "Ebola in Uganda – update". WHO. Retrieved 2012-08-10.
- ^ "DRC Confirms Ebola Outbreak". Voanews.com. Retrieved 2013-04-15.
- ^ "WHO | Ebola outbreak in Democratic Republic of Congo". Who.int. 2012-08-17. Retrieved 2013-04-15.
- ^ "WHO | Ebola outbreak in Democratic Republic of Congo - update". Who.int. 2012-08-21. Retrieved 2013-04-15.
- ^ "Ebola outbreak in DRC - Disaster News Network". Disasternews.net. 2012-08-22. Retrieved 2013-04-15.
- ^ Ebola virus claims 31 lives in Democratic Republic of the Congo, United States: CBS News, 2012, retrieved 14 September 2012
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7787519, please use {{cite journal}} with
|pmid=7787519instead. - ^ King, John W (April 2, 2008). "Ebola Virus". eMedicine. WebMd. Retrieved 2008-10-06.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15752448, please use {{cite journal}} with
|pmid=15752448instead. - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1126/science.1092528, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1126/science.1092528instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9988175, please use {{cite journal}} with
|pmid=9988175instead. - ^ Weingartl HM, Nfon C, Kobinger G (2013) Review of Ebola virus infections in domestic animals. Dev Biol (Basel). 2013;135:211-218
- ^ a b Growing concerns over 'in the air' transmission of Ebola, United Kingdom: BBC News, 2012, retrieved 16 November 2012
- Bibliography
- Klenk, Hans-Dieter (1999). Marburg and Ebola Viruses (Current Topics in Microbiology and Immunology). Berlin, Germany: Springer-Verlag Telos. ISBN 978-3-540-64729-4.
{{cite book}}: Unknown parameter|month=ignored (help)CS1 maint: ref duplicates default (link) - Klenk, Hans-Dieter; Feldmann, Heinz (2004). Ebola and Marburg viruses: molecular and cellular biology (Limited preview). Wymondham, Norfolk, UK: Horizon Bioscience. ISBN 978-0-9545232-3-7.
{{cite book}}: CS1 maint: ref duplicates default (link) - Kuhn, Jens H. (2008). Filoviruses – A Compendium of 40 Years of Epidemiological, Clinical, and Laboratory Studies. Archives of Virology Supplement, vol. 20 (Limited preview). Vienna, Austria: SpringerWienNewYork. ISBN 978-3-211-20670-6.
{{cite book}}: CS1 maint: ref duplicates default (link) - McCormick, Joseph; Fisher-Hoch, Susan (1999) [1996]. Level 4: Virus Hunters of the CDC (Limited preview). Horvitz, Leslie Alan ("Updated edition" 3rd ed.). Barnes & Noble. ISBN 978-0-7607-1208-5.
{{cite book}}: Unknown parameter|month=ignored (help)CS1 maint: ref duplicates default (link) - Pattyn, S. R. (1978). Ebola Virus Haemorrhagic Fever (Full free text) (1st ed.). Amsterdam, Netherlands: Elsevier/North-Holland Biomedical Press. ISBN 0-444-80060-3.
{{cite book}}: CS1 maint: ref duplicates default (link) - Ryabchikova, Elena I.; Price, Barbara B. (2004). Ebola and Marburg Viruses – A View of Infection Using Electron Microscopy. Columbus, Ohio, USA: Battelle Press. ISBN 978-1-57477-131-2.
{{cite book}}: CS1 maint: ref duplicates default (link)
External links
- ViralZone: Ebola-like viruses—Virological repository from the Swiss Institute of Bioinformatics
- CDC: Ebola Hemorrhagic Fever—Centers for Disease Control and Prevention, Special Pathogens Branch
- WHO: Ebola haemorrhagic fever—World Health Organization, Global Alert and Response
- Virus Pathogen Database and Analysis Resource (ViPR): Filoviridae
- 3D macromolecular structures of the Ebola virus archived in the EM Data Bank(EMDB)


